Clinical utility of sperm DNA fragmentation testing: concise practice recommendations
Introduction
Male factors are responsible for approximately half of all infertility cases (1). However, with the advent of intracytoplasmic sperm injection (ICSI), little attention was given to evaluation of male partner of infertile couple and conventional semen analysis remained the only pillar in assessment of male patients despite all the pitfalls (2).
The attention to sperm function tests has returned in recent years. Emerging evidence about the role of sperm DNA integrity on fertilization, embryo development, implantation and pregnancy opens a new horizon in clinical andrology (3,4). Sperm DNA fragmentation (SDF) has been correlated with multiple factors mediated by a number of cellular events. Abnormal chromatin packaging during spermatogenesis, and sperm apoptosis during the final stages of spermatogenesis and epididymal transit, and oxidative stress have all been reported as etiologies of SDF (5,6).
While the importance of SDF has been acknowledged in the latest American Urological Association (AUA) and European Association of Urology (EAU) guidelines on male infertility, there seems to be insufficient evidence to support the routine application of SDF testing in the evaluation of infertile male (7,8). Although evidence supporting the use of SDF testing in some clinical scenario is steadily increasing (9-11), specific indications for the test still await further research. Recently, Agarwal et al. proposed practice recommendations on the clinical utility of SDF testing (12). The expert panel summarized the rapid advancements in SDF measurement and structurally presented, in an evidence-based approach, four clinical scenarios where SDF testing is most indicated. The publication serves as a useful reference for infertility specialists in identifying the clinical circumstances in which SDF testing should be of the greatest value.
This review intends to condense the essence of the practice recommendations and serves as a concise guide to the clinical utility of SDF testing. For the first part, an illustrative review about the available SDF tests are presented. In the second part, recommendations on the clinical utility of SDF tests in common clinical conditions are put forward by consensus of the expert panel.
SDF tests
The clinically available tests to measure sperm DNA damage are summarized in Table 1. These tests are generally classified into two types: direct and indirect. While direct tests measure the extent of sperm DNA damage by using probes and dyes, indirect tests assess the susceptibility of DNA to denaturation which occurs more commonly in fragmented DNA (13). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), the sperm chromatin structure assay (SCSA), and the sperm chromatin dispersion (SCD) test are the most commonly used tests to measure SDF and are briefly presented below. Notably, aniline blue and toluidine blue staining measure nuclear decondensation which is generally associated with sperm immaturity (Table 1).
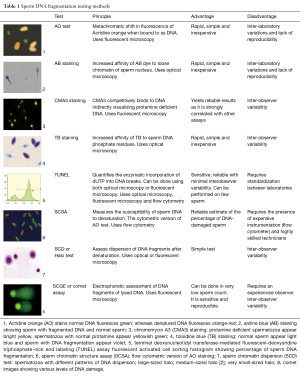
Full table
The SCSA measures the susceptibility of sperm DNA to denaturation. Acridine orange is added after the sample is exposed to heat or acids. The nucleic acid-selective cationic fluorescent dye interacts with double or single strand DNA breaks resulting in a metachromatic shift. The use of flow cytometry allows a large number of cells to be read rapidly and robustly (14). A standardized protocol of SCSA minimize inter-laboratory variation and represents an advantage of the technique. A clinical threshold of SDF index of 30% has been proposed for SCSA test (14). However, the requirement of expensive instrument and highly skilled technicians are the main obstacles for its widespread use (12).
TUNEL detects the incorporation of dUTP into double or single strand DNA breaks via an enzymatic reaction. The fluorescent nucleotides are evaluated with a standard fluorescence microscope or flow cytometry (15). The lack of strict standardization leads to difficult comparison of results among different laboratories and multiple cut-off values have been suggested by various studies (16). On the other hand, the highly specific and reliable assay with minimal inter-observer variability makes TUNEL the preferred technique of SDF testing (17).
Sperm with non-fragmented DNA following acid denaturation and removal of nuclear proteins forms a characteristic halo of dispersed DNA loops. Therefore, the lack of halo in sperm with fragmented DNA forms the basis of the SCD test, also known as the Halo test (18). The test does not require complex instrumentation but is prone to inter-observer variability due to the nature of subjective assessment under microscope.
Measurement of SDF provides a more comprehensive assessment of fertility status in general compared with conventional semen parameters (19,20). Further studies are required in elucidating the exact nature of sperm DNA damage picked up by various SDF tests. Refinement of test protocols and cut-off values for the tests will also better improve the precision of the techniques and decrease inter-laboratory variations.
Indications for SDF testing
Clinical varicocele
The negative impact of clinical varicocele on semen parameters and overall pregnancy rate are well known (21). However, a substantial number of affected men are able to conceive without difficulties and better patient selection for varicocele repair is essential. The presence of varicocele leads to venous stasis and oxidative stress which is accepted as an important mediator in the development of SDF and testicular dysfunction (22). The association between varicocele and SDF in both infertile and fertile men has been reported (23). Moreover, the effect of varicocelectomy in reducing SDF and possibly improving natural conception is supported by a few studies (24,25). On the other hand, the relationship between SDF and varicoceles of different grades is unclear. While an improvement in SDF after varicocele repair is more consistently reported in clinical grade 2 and 3 varicoceles (26,27), the clinical utility of SDF testing in low grade varicocele is less reported (28). Along the same lines, the role of SDF testing in men with large varicoceles and otherwise so-called normal semen analysis is poorly studied.
The clear association between varicocele and SDF, and the reversible nature of SDF by varicocelectomy provide evidence to support the potential value of SDF testing in patient selection for varicocele repair. The additional information offered by SDF tests is particularly valuable when the decision to varicocele ligation is difficult. SDF testing may allow clinicians to better identify surgical candidates in men with clinical varicocele and borderline to normal semen parameters (Table 2). In summary, SDF testing is recommended in men with grade 2/3 varicocele with normal conventional semen parameters and in patients with grade 1 varicocele with borderline/abnormal conventional semen parameters (Table 3, grade C recommendation).
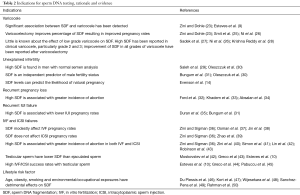
Full table

Full table
Unexplained infertility/recurrent pregnancy loss/intrauterine insemination (IUI) failure
Unexplained infertility is thought to occur in 10-30% of infertile couples (51). It may signify the limitations of semen analysis in identifying the underlying etiologies and a search for new diagnostic tools is needed (52). The role of SDF as an independent predictor of male fertility status has been demonstrated (30,31). It is supported by an observation of impaired sperm DNA integrity in a proportion of men with unexplained infertility and normal semen parameters (29,30). Furthermore, SDF has been shown to be an invaluable prognostic tool of natural pregnancy and IUI success (14,31,35). A few studies also demonstrated a significantly higher SDF in couples with recurrent pregnancy loss (RPL) than controls (32-34).
As a result, it is reasonable to offer SDF testing in couples with RPL and the result may reveal the underlying etiology. SDF tests also represents an option prior to initiating IUI in view of the significant association between high SDF and lower IUI pregnancy rate (Table 2). A high SDF in couples with RPL or IUI failure may suggest the use of in vitro fertilization (IVF) or ICSI as the next treatment step (Table 3, grade C recommendation).
IVF and/or ICSI failure
The relationship between SDF and outcomes of IVF/ICSI has been extensively studied. A modest but significant association between high SDF and lower pregnancy rates in IVF has been summarized by systematic reviews (36,37). The major criticism of the studies in the topic is related to the heterogeneous study design. Multiple potential confounding factors which may affect outcome measures were also not controlled. In fact, female factors contribute significantly to outcomes of assisted reproductive technology (ART) and the effect of SDF on IVF outcome is more pronounced in patients with reduced ovarian reserve (38) thus indicating that the oocyte has some capacity to repair DNA damage. In contrary, high SDF seems to have little influence on ICSI outcomes (36,39). Nevertheless, compelling evidence suggests a correlation between high SDF and pregnancy loss after both IVF and ICSI (36,40-43).
Several treatment strategies, including oral antioxidant, frequent ejaculation, and sperm selection techniques have been proposed to minimize the deleterious effect of high SDF on ART outcomes with varying success. While reduction of SDF has been demonstrated, the treatment effect on ART outcomes is largely uncertain. The use of testicular sperm represents a more promising strategy. It is believed that most SDF occurs during epididymal transit (53) and a significantly lower level of SDF has been found in testicular sperm than ejaculated sperm (44,54). Higher success rates in IVF/ICSI using testicular sperm has been reported in recent studies (10,44,45).
SDF testing can provide useful prognostic information on subsequent ART cycles in patients with recurrent ART failure (Table 2). The use of testicular sperm rather than ejaculated sperm in ICSI may be beneficial in men with oligozoospermia, high SDF and recurrent IVF failure (Table 3, grade B,C recommendation).
Borderline abnormal (or normal) SA with risk factors
Modifiable lifestyle factors exert significant impact on SDF by inducing oxidative stress. Various chemicals in cigarette are found to cause sperm DNA damage (55,56). Indeed, higher SDF has been consistently demonstrated in smokers compared to non-smokers (57). Smoking has also been associated with impaired fertilizing capacity and risk of infertility (58,59). Obesity is another important risk factor of male infertility and abnormal semen parameters may occur due to a number of mechanisms (46). While a few reports did not find an association, larger studies reported a positive correlation between body mass index and SDF (47,60,61). Occupational and environmental exposure is another possible link to male infertility. Exposure to environmental chemicals, organochlorine pollutants and bisphenol A may alter sperm DNA integrity to different degrees (48-50).
SDF tests should be offered to infertile men with evidence of exposures to pollutants or found to have modifiable lifestyle risk factors (Table 2). The test result can reinforce the importance of lifestyle change, predict fertility and monitor patient’s response to risk factor modifications (Table 3, grade C recommendation).
Conclusions
Sperm DNA integrity is essential for human reproduction. Extensive research over the last two decades revealed the significant correlation between SDF and the chances of conception achieved naturally or by ART. SDF testing provides complementary information to semen analysis and both tests should be used in combination for a comprehensive assessment of infertile men. While more studies are needed in clarifying the role of SDF testing in clinical practice, there is currently evidence supporting the use of SDF testing in specific clinical scenarios, including varicocelectomy candidates, couples with recurrent pregnancy loss, patients with unexplained infertility, couples with failed assisted reproduction, and infertile men with exposure to modifiable lifestyle risk factors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resi-dent population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6. [Crossref] [PubMed]
- Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol 2014;40:443-53. [Crossref] [PubMed]
- Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male in-fertility. Hum Reprod Update 2003;9:331-45. [Crossref] [PubMed]
- Lewis SE, Aitken JR, Conner SJ, et al. The impact of sperm DNA damage in assisted con-ception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online 2013;27:325-37. [Crossref] [PubMed]
- Moustafa MH, Sharma RK, Thornton J, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod 2004;19:129-38. [Crossref] [PubMed]
- Gosálvez J, López-Fernández C, Fernández JL, et al. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotech Fertil 2015;4:1-16. [Crossref]
- Jarrow J, Sigman M, Kolettis PN, et al. The optimal evaluation of the infertile male: best practice statement reviewed and validity confirmed 2011. Available online: http://www.auanet.org/guidelines/male-infertility-optimal-evaluation-(reviewed-and-validity-confirmed-2011)
- Jungwirth A, Diemer T, Dohle GR, et al. Guidelines on male infertility. Available online: https://uroweb.org/guideline/male-infertility
- Esteves SC, Gosálvez J, López-Fernández C, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with vari-cocele-associated infertility. Int Urol Nephrol 2015;47:1471-7. [Crossref] [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive out-come in oligozoospermic men with high sperm DNA fragmentation undergoing intracyto-plasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril 2016;105:58-64. [Crossref] [PubMed]
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation test-ing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Majzoub A, Esteves SC, Gosálvez J, et al. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl 2016;18:205-12. [Crossref] [PubMed]
- Evenson DP, Jost LK, Marshall D, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-49. [Crossref] [PubMed]
- Sun JG, Jurisicova A, Casper RF, et al. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997;56:602-7. [Crossref] [PubMed]
- Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril 2014;101:58-63.e3. [Crossref] [PubMed]
- Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-36. [Crossref] [PubMed]
- Fernández JL, Muriel L, Rivero MT, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 2003;24:59-66. [PubMed]
- Evenson DP, Jost LK, Baer RK, et al. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol 1991;5:115-25. [Crossref] [PubMed]
- Oleszczuk K, Giwercman A, Bungum M. Intra-individual variation of the sperm chromatin structure assay DNA fragmentation index in men from infertile couples. Hum Reprod 2011;26:3244-8. [Crossref] [PubMed]
- Jarow JP. Effects of varicocele on male fertility. Hum Reprod Update 2001;7:59-64. [Crossref] [PubMed]
- Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online 2006;12:630-3. [Crossref] [PubMed]
- Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril 2011;96:1283-7. [Crossref] [PubMed]
- Wang YJ, Zhang RQ, Lin YJ, et al. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online 2012;25:307-14. [Crossref] [PubMed]
- Smit M, Romijn JC, Wildhagen MF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 2013;189:S146-50. [Crossref] [PubMed]
- Ni K, Steger K, Yang H, et al. Sperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligation. J Urol 2014;192:170-6. [Crossref] [PubMed]
- Sadek A, Almohamdy AS, Zaki A, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril 2011;95:1705-8. [Crossref] [PubMed]
- Krishna Reddy SV, Shaik AB, Sailaja S, et al. Outcome of varicocelectomy with different degrees of clinical varicocele in infertile male. Advances in Andrology 2015. Available online: http://dx.doi.org/ [Crossref]
- Saleh RA, Agarwal A, Nada EA, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 2003;79 Suppl 3:1597-605. [Crossref] [PubMed]
- Oleszczuk K, Augustinesson L, Bayat N, et al. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013;1:357-60. [Crossref] [PubMed]
- Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007;22:174-9. [Crossref] [PubMed]
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2:76-83. [PubMed]
- Khadem N, Poorhoseyni A, Jalali M, et al. Sperm DNA fragmentation in couples with unexplained re-current spontaneous abortions. Andrologia 2014;46:126-30. [Crossref] [PubMed]
- Absalan F, Ghannadi A, Kazerooni M, et al. Value of sperm chromatin dispersion test in couples with unexplained recurrent abortion. J Assist Reprod Genet 2012;29:11-4. [Crossref] [PubMed]
- Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122-8. [Crossref] [PubMed]
- Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 2009;30:219-29. [Crossref] [PubMed]
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. [Crossref] [PubMed]
- Jin J, Pan C, Fei Q, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro ferti-lization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril 2015;103:910-6. [Crossref] [PubMed]
- Zhao J, Zhang Q, Wang Y, et al. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic re-view and meta-analysis. Fertil Steril 2014;102:998-1005.e8. [Crossref] [PubMed]
- Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 2011;57:78-85. [Crossref] [PubMed]
- Simon L, Brunborg G, Stevenson M, et al. Clinical significance of sperm DNA damage in assisted re-production outcome. Hum Reprod 2010;25:1594-608. [Crossref] [PubMed]
- Lin MH, Kuo-Kuang R, Li SH, et al. Sperm chromatin structure assay parameters are not related to fer-tilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracystoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril 2008;90:352-9. [Crossref] [PubMed]
- Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908-17. [Crossref] [PubMed]
- Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 2005;20:226-30. [Crossref] [PubMed]
- Pabuccu EG, Caglar GS, Tangal S, et al. Testicular versus ejaculated spermtozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous AET failures. Andrologia 2017.49. [PubMed]
- Du Plessis SS, Cabler S, McAlister DA, et al. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 2010;7:153-61. [Crossref] [PubMed]
- Kort HI, Massey JB, Elsner CW, et al. Impact of body mass index values on sperm quantity and quality. J Androl 2006;27:450-2. [Crossref] [PubMed]
- Wijesekara GU, Fernando DM, Wijerathna S, et al. Environmental and occupational exposures as a cause of male infertility. Ceylon Med J 2015;60:52-6. [Crossref] [PubMed]
- Sánchez-Peña LC, Reyes BE, López-Carrillo L, et al. Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol Appl Pharmacol 2004;196:108-13. [Crossref] [PubMed]
- Rahman MS, Kwon WS, Lee JS, et al. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci Rep 2015;5:9169. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril 2006;86:S111-4. [Crossref] [PubMed]
- Hamada A, Esteves SC, Nizza M, et al. Unexplained male infertility: diagnosis and management. Int Braz J Urol 2012;38:576-94. [Crossref] [PubMed]
- Ollero M, Gil-Guzman E, Lopez MC, et al. Characterization of subsets of human spermatozoa at differ-ent stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod 2001;16:1912-21. [Crossref] [PubMed]
- Moskovtsev SI, Jarvi K, Mullen JB, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril 2010;93:1142-6. [Crossref] [PubMed]
- Oyeyipo IP, Maartens PJ, du Plessis SS. In vitro effects of nicotine on human spermatozoa. Andrologia 2014;46:887-92. [Crossref] [PubMed]
- Perrin J, Tassistro V, Mandon M, et al. Tobacco consumption and benzo(a)pyrene-diol-epoxide-DNA adducts in spermatozoa: in smokers, swim-up procedure selects spermatozoa with decreased DNA dam-age. Fertil Steril 2011;95:2013-7. [Crossref] [PubMed]
- Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997;56:602-7. [Crossref] [PubMed]
- Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol 2008;20:281-91. [Crossref] [PubMed]
- Yang F, Li L, Chen JP, et al. Couple’s infertility in relation to male smoking in a Chinese rural area. Asian J Androl 2017;19:311-5. [Crossref] [PubMed]
- Dupont C, Faure C, Sermondade N, et al. Obesity leads to higher risk of sperm DNA damage in infer-tile patients. Asian J Androl 2013;15:622-5. [Crossref] [PubMed]
- Chavarro JE, Toth TL, Wright DL, et al. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010;93:2222-31. [Crossref] [PubMed]


