A step-by-step guide to office-based sperm retrieval for obstructive azoospermia
Introduction
One of the more gratifying procedures in andrology is surgical sperm retrieval. As surgeons, we play a critical role in helping a man become a biologic father when he otherwise would not be able. Because fertility is already a proposition of odds and optimization, we must, as male reproductive surgeons, be prepared to provide the couple the best possible odds of finding viable sperm at the time of sperm retrieval. Frequently, in our practices, we see azoospermic men who have been told by other healthcare professionals that their only hope for pregnancy is with donor sperm. When a diagnosis of obstructive azoospermia (OA) is made, a variety of surgical options exists for treatment. With proper patient selection and surgical technique, it is almost always possible to surgically extract sperm from these azoospermic men to initiate a pregnancy in combination with in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). From spinal cord injured men to men with histories of vasectomy, one must consider each case of OA individually to determine the best surgical approach in order to maximize the quantity and quality of sperm retrieved.
The purpose of this chapter is to guide male reproductive surgeons through the patient selection process, the proper surgical techniques for epididymal sperm retrieval and testicular sperm retrieval for OA, and the management of complications of these office-based sperm retrieval techniques for OA. We also will introduce a minimally invasive modification of open epididymal sperm retrieval that we believe is the optimal technique for sperm retrieval for OA.
Patient selection
As with any surgical procedure, patient selection is critical to successful sperm retrieval. For example, one should never consider epididymal sperm retrieval in men with non-OA (NOA), as the chances of finding sperm in the epididymis and not in the ejaculate is exceedingly rare. To that end, we must first categorize the patient into OA or NOA. If a man has OA, determining the etiology of his OA is important. Is he post vasectomy, and has the couple elected sperm retrieval with IVF instead of vasectomy reversal? If so, he may be a perfect candidate for percutaneous epididymal sperm aspiration (PESA) or our novel technique we will describe later. If a man has congenital bilateral absence of the vas deferens (CBAVD), he may need more extensive exploration of the epididymis, or some may require a testicular sperm retrieval.
There are critical pieces of information needed prior to bringing a patient to the operating room. Just as with any patient encounter, a male reproductive interview must include a detailed history including all medications, supplements and trans-dermal preparations, a full physical exam focusing on the genitals, and appropriate laboratory work-up and focused imaging (1).
Critical points in the history include childhood illnesses, vaccination history, trauma to the pelvis, infectious disease, and current and former medications. Men with a history of pediatric or adolescent cancer may have received chemotherapy which could severely impair spermatogenesis and render them with NOA. Other men may have endocrine disorders that could benefit from hormone modulation prior to sperm extraction. Men on testosterone therapy will have suppressed pituitary secretion of gonadotropins and therefore severely impaired sperm production. Some men on testosterone therapy use a transdermal preparation and will not even disclose that on their medication list. This scenario must be addressed prior to sperm retrieval, as exogenous testosterone can significantly impair spermatogenesis (2), even in a patient with OA (3).
Size and consistency of the testicles are the most important aspects of the preoperative assessment. Men with NOA will likely have smaller testicles than men with OA. We also like to assess the firmness of the epididymis. A plump, indurated epididymis is a welcomed finding on physical exam, as it predicts a successful epididymal sperm retrieval. Small or flat epididymes tend to not have great sperm yields in cases of OA, in our experience. If a man is post-vasectomy and has a sperm granuloma, the chance of finding motile sperm in the vas deferens or distal epididymis increases to nearly 100% (4).
The critical laboratory studies needed prior to sperm retrieval for patients with a working diagnosis of OA, other than preoperative blood work the anesthesiologist may require, include a morning serum total testosterone and follicle stimulating hormone (FSH). Even a patient evaluated for vasectomy reversal should have this basic laboratory assessment of testicular function prior surgery, especially in older men as spermatogenesis can taper off in men over 50. If a patient has either very low FSH less than 2 mIU/mL or elevated FSH greater than 6 mIU/mL, further evaluation and treatment may be necessary to optimize his endocrine function and thus spermatogenesis prior to surgery.
The step-by-step guide
The following section will elucidate a step-by-step approach to sperm retrieval techniques for OA and also describe a novel variation of a well-known technique the authors prefer for men with OA. A description of the advantages and disadvantages of each of these techniques is available in Table 1.
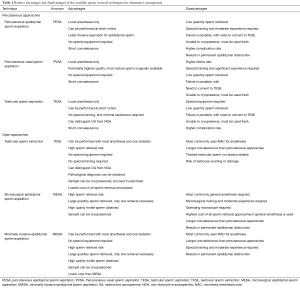
Full table
Anesthesia for office-based sperm retrieval for OA
All office-based sperm retrieval procedures must receive adequate local anesthesia. When the local anesthetic is performed well, the non-anxious patient will tolerate a testicular sperm aspiration (TESA), PESA, testicular sperm extraction (TESE), and minimally invasive epididymal sperm aspiration (MIESA) with local anesthesia alone. Depending on patient factors, cost, and patient and surgeon preference, sperm retrieval in the office may also include general anesthesia, or preferably, monitored anesthesia care (MAC) also known as conscious sedation or twilight anesthesia. While a majority of sperm retrievals are done with local anesthesia plus MAC in these authors’ respective institutions, it should be noted that MAC is used for patient comfort primarily and is not absolutely necessary for a successful outcome or a comfortable and satisfied patient. These authors generally prefer to have an anesthesiologist present for MAC to administer a continuous propofol infusion with intermittent dosing of fentanyl.
When local anesthesia is used alone, oral sedation and oral antibiotics should be considered to be provided prior to the procedure. The authors prefer an oral sedation combination regimen of lorazepam 2 mg and hydrocodone 5 mg to be taken 2 h prior to the procedure. Appropriate training in providing office-based moderate sedation should be acquired by the surgeon, and appropriate monitoring of the patient by the nursing staff and reversal agents should be available. Additionally, oral antibiotics and oral non-steroidal anti-inflammatory drugs (NSAIDs) are started the day prior. According the American Urological Association Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis, for a clean open case where the urinary tract is not entered, a single dose of a first generation cephalosporin is recommended (5). The authors prefer either oral cephalexin starting the day prior to the procedure, or intravenous cefazolin given just prior to the incision, depending whether intravenous access is obtained for a procedure under MAC. A randomized, double-blind, placebo-controlled trial of 34 patients who underwent open sperm retrieval demonstrated significant improvements in post-operative pain and narcotic use when twice daily oral celecoxib 200 mg was initiated the day prior to surgery and continued for a total of 1 week versus placebo (6). The authors of this review currently prefer meloxicam instead of celecoxib due to cost and local insurance coverage, but the importance of perioperative NSAIDs can not be overstated.
For adequate local anesthesia during sperm retrieval, a well-performed spermatic cord block must be used in addition to a peri-incisional and superficial pudendal block. The choice of local anesthetic is by surgeon preference. Typically, either lidocaine or bupivacaine is used for local anesthesia in urologic surgery. For testicular surgery, it is recommended to avoid epinephrine so there is no risk of tissue ischemia as the testicle is an end-organ. Lidocaine has a quick onset and relatively short duration, while bupivacaine has a slightly slower onset and is longer-acting. These authors prefer a combination of lidocaine and bupivacaine. When combined, an injectable solution of lidocaine and bupivacaine has an onset of less than 30 seconds and a duration of approximately 7 h (7). Care should be taken to inject less than the maximum of each, 4 mg/kg of lidocaine without epinephrine and 2 mg/kg of bupivacaine without epinephrine. For example, for a 70-kilogram patient, the maximum dose of lidocaine 1% without epinephrine is 28 mL and bupivacaine 0.25% without epinephrine is 56 mL. The typical sperm retrieval procedure requires approximately 10–20 mL of a 1:1 mixture of plain lidocaine 1% and plain bupivacaine 0.25% without epinephrine for adequate local anesthesia.
A spermatic cord block is performed according to the initial technique described by Wakefield and Elewa (8). Using a 10 mL syringe and 25 gauge, 1.5 inch needle, a 1:1 mixture of lidocaine 1% and bupivacaine 0.25% is infiltrated directly into the high scrotal spermatic cord below the external inguinal ring. For a spermatic cord block, the non-dominant hand grabs the vas deferens with the thumb and index finger, effectively elevating the entire cord between the thumb and index finger, with the vas deferens maintained posteriorly and the proper cord maintained anteriorly and tightly against the scrotal skin. Rather than the quick jabbing maneuver of rapidly passing the needle directly into the center of the structure being anesthetized followed by applying negative suction to the syringe to check if a vascular structure was entered, the authors prefer to continuously infiltrate the anesthetic as the needle is being slowly advanced toward and then into the cord, allowing the anesthetic solution to hydro-dissect away the vessels in its path. While fixating the cord in between the thumb and index finger, approximately three passes of the needle are made into the cord in a fan-like distribution, followed by subcutaneous infiltration lateral to the cord along the scrotal-inguinal plane to anesthetize the superficial branches of the pudendal nerve. A total of approximately 10 mL of anesthetic is used during the spermatic cord and superficial pudendal nerve block. After the initial cord block, a peri-incisional block along the planned skin incision is anesthetized using the same syringe, needle, and solution of lidocaine and bupivacaine as a subcutaneous skin block.
Percutaneous approaches to sperm retrieval for OA
Percutaneous approaches to sperm retrieval for OA are tempting due to the perceived ease in achieving sperm, perceived lower complication rate, and lack of need of conscious or oral sedation. In addition, the lack of specialized equipment required, such as an operative microscope or microsurgical instrumentation, and the ability to perform the procedure with minimal training, make percutaneous approaches more available, particularly at short notice. The disadvantages of percutaneous approaches include a low yield of sperm retrieved compared with open approaches making it less amenable to cryopreservation and thawing, the occasional failed percutaneous retrieval with severe consequences that could include a complete failure of the fresh IVF cycle depending on a successful sperm retrieval, and the lack of ability to schedule the procedure due to the need for coordination with the IVF cycle. In the current era of IVF, sperm retrieval for OA by most fellowship-trained male fertility specialists for cryopreservation involves an open approach, while fresh sperm obtained in coordination with an IVF cycle for couples in which the male partner has OA is more likely to occur via a percutaneous approach. For the experienced fellowship-trained male fertility specialist, a percutaneous approach is a reasonable manner to obtain fresh sperm either in coordination with IVF or if sperm is needed at short notice, so discussion of the techniques is imperative.
The commonly performed percutaneous approaches currently include TESA and PESA. TESA is a needle aspirate of seminiferous tubules most often containing only non-motile or immature sperm, while PESA is a needle aspirate of the head of the epididymis for attempted retrieval of more mature, motile sperm.
PESA
After the initial description of epididymal sperm aspiration in 1985 by Temple-Smith et al. (9), the first described percutaneous sperm retrieval in 1994 by Craft and Shrivastav also targeted epididymal sperm, with what is now known as a PESA (10). Several contemporary series have recently been published of patients who underwent PESA (11-14). Glina and colleagues reported a sperm retrieval rate of 82% in 65/79 patients who underwent PESA, while complications were not reported (12). Esteves et al. reported an overall sperm retrieval rate of 78% in 146 men with OA who underwent PESA. The sperm parameters retrieved were not reported, while the reported complication rate of PESA was 3.4% and included pain, hydrocele, infection, and swelling. The overall live birth rate, which included those who underwent PESA as well as rescue TESA, was 35% (11). Kovac et al. retrospectively reported a perfect 100% sperm retrieval rate for 68 patients undergoing PESA between 2002 and 2010. While quantitative sperm parameters and complications were not reported, the pregnancy rate reported with PESA was 48.6% (14). Hao and colleagues reported a 51% sperm retrieval rate in 96 patients who underwent PESA, although patients with testicular atrophy and elevated FSH were included, possibly diluting the sperm retrieval rate if not all patients had OA. Again, complications were not reported (13). The variability in reported data for PESA underscores the technical difficulty of the procedure; while experience prevails, a lack of experience could result in substantial difficulty for the surgeon and patient.
PESA is performed with local anesthesia, with or without oral sedation. MAC or general anesthesia are typically not necessary. A spermatic cord and skin block is performed as described above. A 21- or 23-gauge butterfly needle is connected to a 20 mL syringe and primed with 1 mL of sperm wash medium. The needle is passed into the upper scrotum through an anesthetized site, and then maximum suction is applied to the syringe and a hemostat is placed across the butterfly needle’s tubing to hold the vacuum. While maintaining the butterfly needle within the scrotum, the head of the epididymis is then positioned within the thumb and index finger, and the needle is passed into the head of the epididymis. Once entered, the hemostat is released, allowing the vacuum to then pull sperm into the tubing. The needle is moved back and forth multiple times without withdrawing it from the skin in order to withdraw the maximum amount of sperm into the tubing and syringe. The sperm are placed on a slide for evaluation by the embryologist. Because adequate sperm for cryopreservation is rarely obtained, the sample is often used immediately for IVF and ICSI. After the needle is withdrawn, it is necessary to apply direct pressure to the site to reduce the risk of hematoma.
For some reproductive urologists routinely performing percutaneous sperm retrieval techniques for OA, PESA is attempted first, and if unsuccessful, “rescue TESA” is the second option which generally has a higher sperm retrieval rate, albeit much lower quantity and quality of sperm.
TESA
It should first be noted that a percutaneous TESA can either be diagnostic or therapeutic. These authors have found TESA to have greater utility as a diagnostic procedure to determine the presence or absence of spermatogenesis in an azoospermic patient with indeterminate clinical findings of OA versus NOA. Such a patient may have had relatively recent exposure to a gonadotoxin such as exogenous testosterone, other clinically indeterminate findings suggesting possible obstruction, or may have mild testicular atrophy with borderline high serum FSH levels or borderline low total testosterone levels. Because TESA can easily be done without oral or conscious sedation, it can quickly provide an answer to the occasional difficult question of OA versus NOA, providing the surgeon more accurate counseling and treatment options for how to move forward in preparation for formal sperm retrieval prior to or concurrent with the IVF cycle. In the case of a short notice call from the reproductive endocrinologist that a semen sample from a patient previously known to have sperm present in the semen is unable to be obtained on the day of oocyte retrieval, for example, TESA is an excellent therapeutic option to have in the armamentarium for a quick and reliable method of sperm extraction under local anesthesia for the patient with known spermatogenesis. TESA can be used as a primary approach for OA, or, as mentioned above, as a secondary option known as a “rescue TESA” in the case of a failed PESA.
Percutaneous TESA to extract sperm for IVF was first described in Israel by Lewin and colleagues in 1996 (15). TESA is usually performed using an 18-gauge 1.5-inch needle fixed into a 10-mL syringe loaded into a fine needle aspiration gun and primed with approximately 1 mL of sperm wash media. These authors use a reusable Cameco syringe pistol device, although disposable devices are available. Another previously described technique of TESA involves using a smaller butterfly needle and hemostat to maintain suction during the aspiration (16), similar to the description of PESA above. Because the amount of sperm retrieved is lower with the smaller needle and less vacuum, these authors prefer the 18-gauge needle on the Cameco pistol syringe. After a spermatic cord and skin block as described above, the testicle is firmly grasped by the assistant with the epididymis maintained posteriorly. Significant caution must be taken to avoid injury to the epididymis during TESA. The scrotal skin is stretched tight and the needle is passed directly into the center of the mid anterior testis before the vacuum is maximally created with the pistol syringe. Maintaining vacuum suction, the needle is then gently moved back and forth in a saw-like pattern, maintaining the needle within the testicular parenchyma. After approximately 10 passes, the needle is withdrawn while still maintaining suction. Tubules will hopefully follow the needle out, and these tubules are lifted up with forceps, and snipped and excised at the level of the skin. The syringe is then removed from the pistol and the aspirate is sprayed into a glass petri dish for the embryologist to assess. Occasionally enough tissue is received to send one or two tubules for pathologic evaluation as well.
Sperm retrieval rates with TESA are generally high, as the outcomes from TESA have been reported in several series. In a publication from 2016, Jensen et al. reported a sperm retrieval rate of 100% in 82 men with OA, with a complication rate of 3% (17). Barring procedural complications, sperm retrieval rates with TESA for OA should in fact be close to 100%, although a less experienced surgeon with the technique must not expect such perfect results.
Percutaneous vasal sperm aspiration (PVSA)
PVSA was initially described in 1997 in six patients with OA secondary to abdominal vasal obstruction or ejaculatory duct obstruction, of which four patients had successful retrieval of motile sperm. One pregnancy using this vasal sperm was reported from a total of four intrauterine insemination (IUI) cycles in three couples (18). The PVSA was later was reported again by the same Chinese authors in 2003 among a larger series of 26 patients with anejaculation. These authors reported an impressive 100% sperm retrieval rate, and 19 pregnancies for a 73.1% pregnancy rate after 34 IUI procedures (19). Because it is not widely performed, a standard technique of PVSA is relatively unknown. Qiu and colleagues review the technical details of their technique in the only publications describing the PVSA procedure (18,19). The technique is similar to that of the more commonly known vasography technique. After appropriate local anesthesia as previously described, the vas deferens is delivered in similar fashion to the well described no-scalpel vasectomy (20). Qiu et al. describe using a 21-gauge sharp needle to first pierce the vas in the direction of the epididymis, followed by passage of a 23-gauge blunt tip needle introduced through the sharp needle. The blunt-tipped needle is then connected to a 10-mL syringe primed with 1 mL of sperm wash medium, of which 0.2–0.3 mL is pushed into the vas deferens, before gentle suction aspiration is applied to the syringe (19). An alternative technique is described by Khurana and Sabanegh (21). These authors describe the importance of planned microsurgical reconstruction with vasovasostomy or epididymovasostomy at the time of PVSA, however certain cases may be considered for PVSA alone. The technique described by Khurana and Sabenegh is performed with the vas deferens being exposed through a conventional paramedian vasectomy incision of 1.5 cm and then partially transected for the aspiration under the guidance of an operating microscope. After aspiration, if reconstruction is not planned, the vasotomy is closed with interrupted 10-0 and 9-0 nylon in the standard fashion of a vasovasostomy. Khurana and Sabanegh describe their technique in their 2013 review paper, but do not reference or provide any results for the PVSA procedure (21). As an office-based procedure with similar morbidity as a vasectomy, the technique is promising; however, while the reported results of PVSA of the two Qiu studies are encouraging, those authors reported findings in 1997 and 2003 which have yet to be replicated or reported in similar fashion by others in the literature.
Conventional open approaches to sperm retrieval for OA
TESE
Perhaps the most well-known sperm retrieval method is the TESE. Because the procedure is identical to a testicular biopsy, it is familiar to most urologists. Additionally, because it doesn’t require the use of an operating microscope or microsurgical training, it is widely available. Most importantly, a TESE for OA virtually guarantees a successful sperm retrieval, with the majority of reports on TESE for OA demonstrating a 100% sperm retrieval rate. Because of its widespread, long-term acceptance as the gold standard method for sperm retrieval for OA, newer techniques are compared against it while studies reporting outcomes for TESE specifically for the population of patients with OA are lacking.
Traditional dogma informs the surgeon that testicular biopsy is indicated in azoospermic men with normal volume testes, palpable vasa deferentia, and normal or near-normal serum FSH levels. The TESE in this scenario may therefore serve several purposes: it may distinguish between OA and NOA, obtain a tissue diagnosis, as well as extract substantial sperm for cryopreservation. A truly diagnostic biopsy is now rarely performed in lieu of a TESE which serves all of these additional purposes. For this reason, it is vitally important to perform a testicular biopsy in this scenario in a center with the ability to quantify and cryopreserve the tissue and sperm. Within the framework of distinguishing OA from NOA, another benefit of the TESE is obtaining tissue for pathologic diagnosis if no sperm is found. Any other method of percutaneous or open sperm retrieval which fails to identify sperm may be converted to a TESE with relative ease, and the ability to maneuver the conversion to a TESE should be made feasible within the chosen operative setting.
While rare, diagnostic biopsies are often performed bilaterally; a TESE, however, is most often a unilateral case. If one testis is larger, healthier, or without a varicocele, that side is preferred; most commonly, using these criteria, the right side would be favored. However, if the patient has previously had testicular surgery, trauma, or epididymitis on one side, these situations should be avoided where possible. The surgeon and patient should both be prepared for a possible bilateral procedure should the need arise.
A TESE can be performed with oral sedation with local anesthesia or MAC in the office setting. Patient and surgeon factors may dictate the preferred anesthetic approach. While magnification is not necessary, the authors find loupe magnification particularly helpful. The case should always begin with a spermatic cord, superficial pudendal, and peri-incisional local anesthetic block as described above, both for anesthesia during the case as well as for post-operative analgesia. After adequate anesthesia is achieved, if a unilateral procedure is planned, a 1 cm upper hemiscrotal transverse incision is made within a scrotal rugation while the assistant carefully positions the testicle beneath the planned incision with the skin kept tight across the testicle and the epididymis positioned posteriorly. If a bilateral procedure is planned, a midline incision may be utilized. After the incision, the dartos fascia is opened with electrocautery and the tunica vaginalis is opened sharply. To aid in later closure, small mosquito hemostats left on the tunica vaginalis at the initial entry point can be helpful after opening it the length of the incision, and then an eyelid retractor is placed. Delivery of the testicle is not necessary. When the tunica albuginea is exposed, a 5-0 chromic on an S14 needle is pre-placed and the albuginea is incised for 5–10 mm along a mid-pole, anterior, avascular plane. Gentle pressure is applied to the testicle allowing for extrusion of seminiferous tubules. The tubules are freed from the overlying albuginea with gentle sweeps of a iris scissor beneath the edges of the incision, and the tubules are then lifted with forceps and sharply excised. A testicular biopsy for pathology may be taken from the sample prior to mincing the tissue. The tissue is then finely minced with iris scissors within approximately 1 mL of sperm wash medium in a glass petri dish, and a small drop is placed on a slide for evaluation by the embryology staff. Hemostasis is achieved through bipolar electrocautery, and the incision in the tunica albuginea is closed with the pre-placed suture in running fashion. Multiple biopsy sites are often not necessary. The tunica vaginalis, dartos fascia, and skin are closed in running fashion with 3-0 chromic.
Microsurgical epididymal sperm aspiration (MESA)
The use of sperm for IVF obtained from an open epididymal sperm aspiration was reported in 1985 (9), and the addition of an operating microscope for a MESA was originally described in 1988 by Silber and colleagues (22). Later prompting a move toward the popularization of TESE, subsequent research indicated that the more easily extracted testicular sperm do not differ in function from epididymal sperm with respsect to IVF-ICSI outcomes including fertilization (23-25). However, consideration by the contemporary surgeon to extract the highest quantity and quality sperm available is an approach that lends itself to better samples and thus potentially better outcomes, and indeed, a large contemporary study comparing MESA with TESE for OA strongly favored epididymal sperm over testicular sperm (26). In this retrospective study, the largest of its type, 280 men underwent MESA and 94 underwent TESE with subsequent ICSI. The live birth rate was significantly higher after MESA than after TESE (39% versus 24%, P=0.011), and after adjusting for confounding variables, the odds ratio for live birth after MESA compared with TESE for OA was 1.82 (95% CI, 1.05–3.67). The study also showed that fresh versus frozen sperm for both MESA and TESE for OA had no significant difference in outcomes (26). These staggering data challenge the traditional dogma that all surgically extracted sperm function equally; perhaps, obtaining higher quantity, mature, motile sperm is a preferred approach for the obstructed patient.
There are several different techniques for MESA that have been reported (27-30). The conventional MESA is not usually performed in the office but rather in the operating room under general anesthesia. A high transverse hemiscrotal incision, vertical paramedian incision, or midline incision may be utilized. As with the description of TESE above, a unilateral approach to the healthier testicle is most often sufficient. With a conventional MESA, after the vaginalis is opened, the entire testicle is delivered into the wound for full exposure of the testicle and epididymis. The operating microscope is docked and the epididymis is evaluated under 16× to 25× magnification. In the case of prior vasectomy, evaluation of the epididymal tubules for a “blowout”, or transitional change in appearance of the tubular fluid indicating a secondary obstruction, is performed, and sperm are initially targeted proximal to the transition toward the epididymal head for the best motility.
After the epididymis is evaluated with the operating microscope, the traditional MESA as described by Schlegel, Goldstein, and colleagues in various publications (29,30), and more recently by Bernie et al. (27), involves making an epididymotomy in the same fashion as what is used for epididymovasostomy and then drawing the epididymal fluid into a glass capillary tube. The aspirated fluid is then placed onto a slide, mixed with a drop of sperm wash media, and passed off to the embryologist for evaluation for sperm motility. Because sperm gain maturity through epididymal transport, the sperm should be surgically extracted from the distal-most epididymal location where quality motility is observed, although rarely are viable sperm available as distally as the epididymal tail. If an epididymal blowout or transition is not observed, the mid pole of the epididymis is a good starting place. As sites are sequentially checked, the surgeon should move proximally toward the epididymal head until high quality motile sperm are identified for extraction. Of note, the longer the patient has been obstructed, the more likely the head of the epididymis will be the only anatomical epididymal region containing viable, motile sperm. In the case of patients with CBAVD, typically the best place where quality sperm will be found is near the efferent ducts of the head of the epididymis. Often multiple epididymotomy sites are necessary to evaluate before the highest quality motile sperm are located for complete extraction. The experience of performing MESA for OA, particularly when multiple sites are necessary to evaluate before motile sperm are identified, underscores the pitfalls and potential difficulty of PESA where a needle is blindly passed into the epididymis.
The authors of this review prefer a MESA technique which has now been coined in the literature as an “obliterative MESA” by Karpman and Williams (28), although this MESA technique was initially performed and popularized by Larry Lipshultz. The obliterative technique for MESA was developed at Baylor in the mid-1990s after the landmark paper from Schlegel and colleagues was published first describing the glass capillary tube aspiration technique for MESA (29). As described by Lipshultz, while the selective aspiration with the glass capillary tube was innovative, it could limit the yield of the sperm retrieval. The obliterative MESA was developed to maximize the sperm yield in order to obtain enough sperm to cryopreserve the sample in multiple vials (Larry Lipshultz, verbal communication).
The obliterative MESA exhausts all available tubules containing motile sperm in order to maximize the quantity of sperm extracted. An ophthalmic blade is used to make multiple epididymotomies, and while the surgeon squeezes the epididymis to milk out the tubular fluid, the assistant uses a tuberculin syringe with 24 gauge angiocatheter tip primed with sperm wash media to aspirate the fluid. After a site is located with motile sperm, as many tubules as possible proximal to that site are drained and aspirated. When the maximum amount of epididymal fluid is extracted, electrocautery may be used for hemostasis over the epididymotomies. The coagulation current is raised and the cautery is used in a similar fashion to holmium laser coagulation used in renal surgery in a sweeping manner effectively thereby obliterating the epididymis, thus the name. After hemostasis is assured, the tunica vaginalis is closed with 3-0 chromic and the testicle is returned to the scrotum. Dartos fascia and skin are each closed with 3-0 chromic in a running fashion.
The patient should understand that after an obliterative MESA that future surgical reconstruction is not possible. Despite the seemingly greater invasiveness of the obliterative MESA than the traditional MESA, postoperative pain and time to convalescence are minimal and are essentially the exact same between the two techniques.
MIESA—a new technique for sperm retrieval for OA
The ideal sperm retrieval for OA would obtain the maximum quantity of high quality sperm, while minimizing procedural-related risk, complications, and cost. To address these concerns, the authors of this review prefer a MESA technique we call a MIESA. Depending on surgeon preference, MIESA can be pronounced the same as “MESA” or with a distinct pronunciation like “Me-ay-sa”. To our knowledge, this technique has never been described previously, although the “mini-MESA” described by Karpman and Williams (28) and the “mini-micro-epididymal sperm aspiration” described by Nudell et al. (31) are similar. Both the “mini-micro-epididymal sperm aspiration” and the “mini-MESA” utilize a keyhole incision, although the MESA is performed in standard fashion under general anesthesia and using operating microscope.
The unique aspects of the MIESA technique include utilizing a 1 cm keyhole incision in the style of a TESE, not delivering the testicle while only the epididymis is exposed within the window incision, and performing it in the office without the need for general anesthesia or an operating microscope. While any aspiration technique can be used during MIESA, including the selective aspiration of a tubule with the glass capillary tube, we prefer the obliterative MESA technique of aspiration to maximize the amount of sperm retrieved.
The setup and procedure begins in a similar fashion to the office-based TESE described above. Instead of an operating microscope, loupe magnification is recommended. Briefly, oral sedation or MAC, along with local anesthesia, is utilized during the procedure. Extraction from only one testicle is necessary with a MIESA to achieve an excellent yield. A spermatic cord block with superficial pudendal nerve block and peri-incisional skin block are performed after MAC is induced. A 1-cm transverse upper hemiscrotal incision is made. Care is taken to make the incision high in the scrotum to be sure to have the best access to the head of the epididymis. During the incision and initial dartos incision, the assistant must grip the testicle beneath the incision in a way that permits access to the superior aspect of the tunica vaginalis; a mistake often occurred in our early experience where the incision in the tunica vaginalis was too low, making delivery of the head of the epididymis through the window incision difficult. The dartos is opened with electrocautery (if available) and the vaginalis is entered sharply. Two fine hemostats are left on the tunica vaginalis at the intial entry site for later closure before it is opened the length of the incision.
The head of the epididymis is secured with a toothed adson forceps and delivered into the incision. A stay suture is placed in the upper third of the epididymis in the space between the epididymis and the testicle and is used as a traction suture. The traction suture allows for easy manipulation and maintenance of the epididymis into the window incision. For the awake patient not under MAC, if pain is increased with this stimulation, additional local anesthesia may be injected around the fusion of the vaginalis to the posterior epididymis as a focal epididymal block. After the traction suture is placed, an eyelid retractor is positioned. The initial epididymal exposure during MIESA for a patient with CBAVD is demonstrated in Figure 1A. A close-up view, similar to the typical view with loupe magnification, demonstrates the clearly apparent epididymal tubules that can be visualized through the epididymal tunic (Figure 1B).
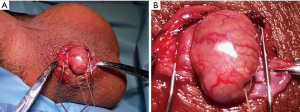
The surgeon then positions his or her thumb and index finger above the eyelid retractor, but beneath the traction suture which can then be elevated to allow for the best grip of the head of the epididymis (Figure 2A). The other fingers of the non-dominant hand can cup and support the testicle. One key to success is a tight epididymal grip with the non-dominant thumb and index fingers which allows for excellent hemostasis as well as milking of the fluid during aspiration. As described above in the section on MESA, because sperm gain maturity through epididymal transport, the sperm should be surgically extracted from the distal-most epididymal location where quality motility is observed, although rarely are viable sperm available as distally as the epididymal tail. If an epididymal blowout or transition is not observed, the mid pole of the epididymis is a good starting place.
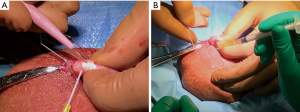
After the grip is secured, the assistant is prepared with a tuberculin syringe and 24 gauge angiocatheter tip primed with about 0.1 mL of sperm wash medium. Note that three of these syringes are prepared so backups are ready on the mayo stand. With the surgeon’s dominant hand, an ophthalmic blade is used to begin puncturing tubules. The authors prefer a 15-degree double beveled straight ophthalmic knife which has the general shape of a #11 scalpel blade but is much finer. While a careful epididymotomy can be made by first carefully coming through the epididymal tunic and then targeting a specific tubule in the typical fashion of epididymovasostomy, these authors have found that to be an unnecessary waste of operative time; the ophthalmic blade can be passed directly through the tunic and into a tubule with a slow stabbing motion directly into the epididymis. As the blade is withdrawn from the epididymal puncture site, the epididymal fluid is then expressed and may be aspirated by the assistant. Fluid from the initial puncture is placed on a slide for evaluation by the embryologist (Figure 2B). Note that multiple sites may need to be evaluated before the highest quality motile sperm are located for complete extraction. As sites are sequentially checked, the surgeon should move proximally toward the epididymal head until high quality motile sperm are identified for extraction.
When quality motile sperm are identified, the surgeon and assistant then begin aspirating all available tubules from that anatomical location and everything proximally toward the head of the epididymis. This process will often be very fast at this point, as fluid will drain rapidly after several nearby epididymotomies are made. During aspiration, care is taken by the assistant not to pull the plunger out of the back of the syringe; once it is close to being full (primarily of air), it is quickly handed off to the scrub nurse who switches it out with a fresh one. The scrub nurse will take the full syringes and empty them into a test tube containing about 3 mL of sperm wash media, then prime the empty syringe and place it back onto the mayo stand as the aspiration is rapidly performed. After a site is initially checked and quality motile sperm are identified, the entire epididymis proximal to that location can usually be drained in under 5 min. If any bleeding occurs from the epididymal tunic, focal bipolar electrocautery may be used for hemostasis.
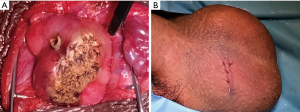
Nearing the conclusion of the obliterative MIESA, a slide is made from the sperm wash media in the test tube which represents the final concentration. Often sperm will be present in a quantity approaching a typical semen analysis; in that case, the aspiration portion of the procedure is concluded. Electrocautery is then used for hemostasis over the multiple epididymotomy sites. The coagulation current is raised and the cautery is used in a sweeping manner over the epididymis (Figure 3A). After hemostasis is assured, the tunica vaginalis is closed with 3-0 chromic. A small hydrocele with local anesthesia is left to bathe the testicle and epididymis as the vaginalis is closed. Dartos fascia and skin are each closed with 3-0 chromic in a running fashion (Figure 3B).
Post-operative instructions for MIESA include that the patient will have a gauze dressing over his incision and will be discharged with a scrotal supporter in place. The patient will have a prescription for narcotic pain medication which may be necessary for 2–5 days post-operatively. The authors restrict patient activity to no strenuous physical activity for a week post-operatively. At one week, if there is no swelling and no pain, men can return to normal physical and sexual activity. These authors do not schedule a routine post-operative visit.
Complications, pitfalls, and anecdotes of sperm retrieval for OA
Clinically significant complications after sperm retrieval are rare; however, in the available literature on sperm retrieval techniques, complications are infrequently reported. Hematoma and infection are the most common complications after scrotal surgery, and they are generally less than 3% for sperm retrieval procedures, but could be higher for percutaneous procedures (32-34). Transient scrotal ecchymoses are common after percutaneous approaches (33), although large scrotal hematomas are relatively infrequent. A 5.4% rate of bleeding-related complications was observed in 2 of 37 patients who underwent combined TESA and TESE in the same setting for NOA (32). In another study, 1 patient out of 46 (2.2%) who underwent conventional TESE had a hematocele and wound infection, while no complications occurred in 100 patients who underwent microdissection TESE (micro-TESE) (34).
A greater percentage of men have subcapsular hematomas after percutaneous sperm retrieval procedures, and most regress by 6 months (34-36). In a prospective study designed to assess testicular damage after TESA, Raviv et al. reported that 2 of 32 patients (6.2%) had transient evidence of testicular damage 6 weeks after the procedure which resolved in both patients by 3 months (35). A similar study of 35 men who underwent TESA found that focal testicular lesions were seen in four out of 61 testes (6.6%) at 3 months; three lesions resolved after 6 months, and all resolved after 9 months. Surprisingly some degree of testicular echogenicity remained unchanged in 50 cases (82%) 3 months after TESA. Lastly, severe post-operative pain was reported by 4 of 35 men (11.4%), but only 1 was found to have an intratesticular hematoma (36).
Understanding that complications happen in surgery, it is even more critical to minimize the complication rate in the field of male fertility where the patients are healthy and essentially have no preoperative disability. Men have high expectations that their surgery will be virtually pain free, precise, and successful. To that end, even though most procedures for sperm retrieval are minor, it is necessary to treat every procedure as a major surgery. Surgical checklists are important to make sure all equipment and supplies are available and ready for every case. For example, the surgeon must be responsible to have the necessary media for sperm storage and transport, as well as the necessary instrumentation and supplies to perform any procedure necessary, from TESE to epididymal sperm aspiration, as one never knows where viable sperm will be identified within a testicle.
One author of this manuscript (Jesse N. Mills) recalls one of the first sperm retrievals he performed at an off-site reproductive medicine clinic, where he brought everything including the microsurgical instrument tray, local anesthetic, and even a post-procedural scrotal supporter. He expected the clinic would have the basics such as a razor and a scalpel. The clinic had neither for the TESE he was about to perform. He likely retains the dubious distinction of performing the first and only no-scalpel TESE with super sharp Jacobsen mosquito through an unshaved scrotum. The importance of preparedness with appropriate supplies and instrumentation can not be overstated.
One of the theoretical advantages to the MIESA technique is direct visualization through the entire procedure. To that end, in the hundreds of procedures these authors have performed, we have not had a single hematoma. This advantage to MIESA also involves additional rotational exposure to the entire testicle if necessary. If sperm are not discovered in the epididymis, the testis is easily repositioned for direct exposure to the entire testicle and therefore, one can easily convert to TESE, or even, in a properly equipped operating suite, micro-TESE if necessary.
The direct visualization of the epididymis and testis is a distinct advantage MIESA has over percutaneous techniques. This allows, with proper loupe magnification, complete assessment of the epididymal tubules (Figure 1B). An author of this manuscript (Jesse N. Mills) had a case where this proved to be very helpful. The patient had unexplained OA, presumably from severe bilateral epididymitis. During the MIESA, dilated and yellow-appearing epididymal tubules were observed in the distal epididymis. Proximally, the tubules appeared white and unobstructed. Aspiration was carefully performed at the distal most aspect of the epididymis near the transition point. After a cycle of IVF that resulted in one live birth and no cryopreserved supernumerary embryos, the couple returned for consult for epididymovasostomy as they were reluctant to pursue IVF for the second child. Because the MIESA technique identified a transition point on one side, the patient subsequently underwent bilateral epididymovasostomy with successful return of motile sperm to the ejaculate.
Complications are more frequent with percutaneous approaches including TESA and PESA, however there are a greater number of better designed studies for these approaches as well. By comparison, open approaches including TESE and MESA have lower complication rates owed to the fact that these procedures are done in more controlled environments with better ability to perform hemostasis, such as electrocautery. In these authors experience, MIESA has the lowest complication rate of any sperm retrieval, owed to its minimally invasive approach and controlled environment. Whatever the approach, the surgeon must be meticulous about hemostasis to prevent a hematoma which is the most common complication of scrotal surgery. The patient should understand that with any approach for sperm retrieval there is a small risk for hematoma, infection, or pain, all of which generally resolve with time if they occur.
Conclusions
This chapter should serve as a reference for both advanced and novice male reproductive surgeons to guide them through every stage of sperm retrieval, including preoperative evaluation, patient selection, procedural techniques, and complications. Success rates of sperm retrieval in cases of OA should be almost 100% if the surgeon is well trained and the patient is well prepared and evaluated. With the incredible advances in IVF combined with innovative surgical treatment for male factor infertility in recent years, OA is no longer a barrier for men to become biologic fathers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith RP, Coward RM, Lipshultz LI. The office visit. Urol Clin North Am 2014;41:19-37. [Crossref] [PubMed]
- McBride JA, Coward RM. Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian J Androl 2016;18:373-80. [Crossref] [PubMed]
- Coward RM, Mata DA, Smith RP, et al. Vasectomy reversal outcomes in men previously on testosterone supplementation therapy. Urology 2014;84:1335-40. [Crossref] [PubMed]
- Boorjian S, Lipkin M, Goldstein M. The impact of obstructive interval and sperm granuloma on outcome of vasectomy reversal. J Urol 2004;171:304-6. [Crossref] [PubMed]
- Wolf JS Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol 2008;179:1379-90. [Crossref] [PubMed]
- Mehta A, Hsiao W, King P, et al. Perioperative celecoxib decreases opioid use in patients undergoing testicular surgery: a randomized, double-blind, placebo controlled trial. J Urol 2013;190:1834-8. [Crossref] [PubMed]
- Collins JB, Song J, Mahabir RC. Onset and duration of intradermal mixtures of bupivacaine and lidocaine with epinephrine. Can J Plast Surg 2013;21:51-3. [Crossref] [PubMed]
- Wakefield SE, Elewa AA. Spermatic cord block: a safe technique for intrascrotal surgery. Ann R Coll Surg Engl 1994;76:401-2. [PubMed]
- Temple-Smith PD, Southwick GJ, Yates CA, et al. Human pregnancy by in vitro fertilization (IVF) using sperm aspirated from the epididymis. J In Vitro Fert Embryo Transf 1985;2:119-22. [Crossref] [PubMed]
- Craft I, Shrivastav P. Treatment of male infertility. Lancet 1994;344:191-2. [Crossref] [PubMed]
- Esteves SC, Lee W, Benjamin DJ, et al. Reproductive potential of men with obstructive azoospermia undergoing percutaneous sperm retrieval and intracytoplasmic sperm injection according to the cause of obstruction. J Urol 2013;189:232-7. [Crossref] [PubMed]
- Glina S, Fragoso JB, Martins FG, et al. Percutaneous epididymal sperm aspiration (PESA) in men with obstructive azoospermia. Int Braz J Urol 2003;29:141-5; discussion 5-6. [Crossref] [PubMed]
- Hao L, Li ZG, He HG, et al. Application of percutaneous epididymal sperm aspiration in azoospermia. Eur Rev Med Pharmacol Sci 2017;21:1032-5. [PubMed]
- Kovac JR, Lehmann KJ, Fischer MA. A single-center study examining the outcomes of percutaneous epididymal sperm aspiration in the treatment of obstructive azoospermia. Urol Ann 2014;6:41-5. [Crossref] [PubMed]
- Lewin A, Weiss DB, Friedler S, et al. Delivery following intracytoplasmic injection of mature sperm cells recovered by testicular fine needle aspiration in a case of hypergonadotropic azoospermia due to maturation arrest. Hum Reprod 1996;11:769-71. [Crossref] [PubMed]
- Gorgy A, Podsiadly BT, Bates S, et al. Testicular sperm aspiration (TESA): the appropriate technique. Hum Reprod 1998;13:1111-3. [Crossref] [PubMed]
- Jensen CF, Ohl DA, Hiner MR, et al. Multiple needle-pass percutaneous testicular sperm aspiration as first-line treatment in azoospermic men. Andrology 2016;4:257-62. [Crossref] [PubMed]
- Qiu Y, Wang S, Yang D, et al. Percutaneous vasal sperm aspiration and intrauterine insemination in the treatment of obstructive azoospermia. Fertil Steril 1997;68:1135-8. [Crossref] [PubMed]
- Qiu Y, Wang SM, Yang DT, et al. Percutaneous vasal sperm aspiration and intrauterine insemination for infertile males with anejaculation. Fertil Steril 2003;79:618-20. [Crossref] [PubMed]
- Li SQ, Goldstein M, Zhu J, et al. The no-scalpel vasectomy. J Urol 1991;145:341-4. [Crossref] [PubMed]
- Khurana KK, Sabanegh ES Jr. Office-based sperm retrieval for treatment of infertility. Urol Clin North Am 2013;40:569-79. [Crossref] [PubMed]
- Silber SJ, Balmaceda J, Borrero C, et al. Pregnancy with sperm aspiration from the proximal head of the epididymis: a new treatment for congenital absence of the vas deferens. Fertil Steril 1988;50:525-8. [Crossref] [PubMed]
- Moghadam KK, Nett R, Robins JC, et al. The motility of epididymal or testicular spermatozoa does not directly affect IVF/ICSI pregnancy outcomes. J Androl 2005;26:619-23. [Crossref] [PubMed]
- Nicopoullos JD, Gilling-Smith C, Almeida PA, et al. Use of surgical sperm retrieval in azoospermic men: a meta-analysis. Fertil Steril 2004;82:691-701. [Crossref] [PubMed]
- Schwarzer JU, Fiedler K, v Hertwig I, et al. Sperm retrieval procedures and intracytoplasmatic spermatozoa injection with epididymal and testicular sperms. Urol Int 2003;70:119-23. [Crossref] [PubMed]
- van Wely M, Barbey N, Meissner A, et al. Live birth rates after MESA or TESE in men with obstructive azoospermia: is there a difference? Hum Reprod 2015;30:761-6. [Crossref] [PubMed]
- Bernie AM, Ramasamy R, Stember DS, et al. Microsurgical epididymal sperm aspiration: indications, techniques and outcomes. Asian J Androl 2013;15:40-3. [Crossref] [PubMed]
- Karpman E and Williams D. Techniques of sperm retrieval. In: Lipshultz LI, Howards SS, Niederberger CS. editors. Infertility in the male. 4th edition, New York: Cambridge University Press, 2009:407-20.
- Schlegel PN, Berkeley AS, Goldstein M, et al. Epididymal micropuncture with in vitro fertilization and oocyte micromanipulation for the treatment of unreconstructable obstructive azoospermia. Fertil Steril 1994;61:895-901. [Crossref] [PubMed]
- Goldstein M, Tanrikut C. Microsurgical management of male infertility. Nat Clin Pract Urol 2006;3:381-91. [Crossref] [PubMed]
- Nudell DM, Conaghan J, Pedersen RA, et al. The mini-micro-epididymal sperm aspiration for sperm retrieval: a study of urological outcomes. Hum Reprod 1998;13:1260-5. [Crossref] [PubMed]
- Friedler S, Raziel A, Strassburger D, et al. Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non-obstructive azoospermia. Hum Reprod 1997;12:1488-93. [Crossref] [PubMed]
- Levine LA, Dimitriou RJ, Fakouri B. Testicular and epididymal percutaneous sperm aspiration in men with either obstructive or nonobstructive azoospermia. Urology 2003;62:328-32. [Crossref] [PubMed]
- Okada H, Dobashi M, Yamazaki T, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol 2002;168:1063-7. [Crossref] [PubMed]
- Raviv G, Levron J, Menashe Y, et al. Sonographic evidence of minimal and short-term testicular damage after testicular sperm aspiration procedures. Fertil Steril 2004;82:442-4. [Crossref] [PubMed]
- Westlander G, Ekerhovd E, Granberg S, et al. Serial ultrasonography, hormonal profile and antisperm antibody response after testicular sperm aspiration. Hum Reprod 2001;16:2621-7. [Crossref] [PubMed]


