Technique considerations and complication management in transurethral resection of the prostate and photoselective vaporization of the prostate
Introduction
The prevalence of lower urinary tract symptoms (LUTS) increases with age from 13–14% in the fifth decade of life to 28–43% after age 60 (1-4), making LUTS one of the most commonly treated conditions by urologists. Surgical options for benign prostatic hyperplasia (BPH) are reserved for men who have inadequate symptomatic relief from medication, or who are unable to tolerate them.
Endoscopic treatment options for LUTS due to BPH (LUTS/BPH) including transurethral resection of the prostate (TURP) and photoselective vaporization of the prostate (PVP) are the mainstay of treatment for men with small to moderate sized glands (30 to 80 cc), which accounts for the majority of men who elect surgical treatment.
While TURP and PVP both have a long history of safety and efficacy for the treatment of UTS/BPH, novel treatment technologies are continuously being introduced. Eventually one of these new technologies may supplant TURP and PVP as the most commonly used treatment for men, but until then the urologist will need to maintain one of these treatment options as the backbone of their surgical practice. In this manuscript we will discuss these two treatment options, technical/intra-operative considerations to optimize outcomes and management of both common and uncommon complications.
TURP
Introduction
TURP is an endoscopic approach to surgically remove the portion of the prostate adenoma encircling the urethra. An electrified wire loop is moved through the tissue to cut the adenoma into strips small enough to be removed via the urethra. TURP was the first endoscopic option for management of LUTS/BPH, and has been a mainstay of urology for well over 50 years in various iterations. Its continued use to today demonstrates both the reliability of the technology coupled with improvements in technique and technology, which have kept it germane to the practicing urologist.
Technical factors
In a monopolar TURP the current is carried through the cutting loop into the tissue (and through the patient) to the grounding pad electrode. This type of cutting current requires a non-ionic irrigant (water, glycine, sorbital) to allow for electroresection. Unfortunately, use of these fluids can be absorbed via the prostate into the systemic blood supply and cause acute dilutional hyponatremia (TUR syndrome).
The migration of monopolar to bipolar energy has produced a considerable improvement in the safety profile of TURP. The use of iso-osmolar saline in bipolar TURP has eliminated the incidence of TUR syndrome completely from the vernacular of urologists. The different energy utilization and localized effects with bipolar TURP has improved hemostatic properties reducing bleeding events and improving visualization during the procedure.
Bipolar resection incorporates both electrodes (active and return) into the working electrified resection loop (5) with energy now concentrated solely at the site of tissue-electrode interaction. Different bipolar systems have different precise mechanisms of action but are defined by the concentration of energy at the site of resection and the ability to resect in iso-osmolar saline. This improvement in the age old TURP has been quickly and widely adapted by urologists.
Intraoperative plan
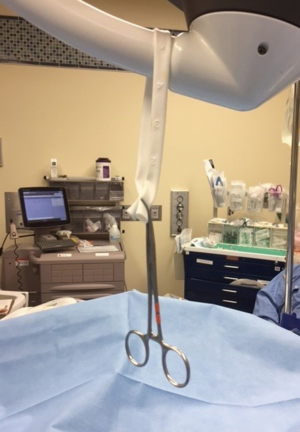
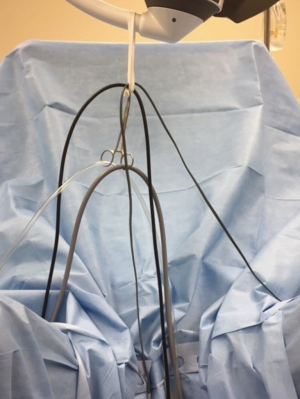
The resectoscope parts should be inspected and made sure to be in correct working order before introducing the scope into the patient. We advocate the use of a “zero gravity” set up of the equipment (Figure 1). Taking a few moments before the case to set this up removes the drag placed on the cords and resection equipment by hanging it centrally over the patient from one of the overhead lights. A penrose is looped around the overhead light and a clamp is used to secure it (Figure 1). The cords and irrigant tubing that run to the resectoscope are then placed through the penrose or clamp (Figure 2) to keep them from pulling off to the side of the patient. This reduces the drag on the cords and in turn frees up the resectoscope to move more effortlessly.
The relevant anatomy should be surveyed at the beginning of the case including identification of the ureteral orifices (UOs) and verumontanum. The bladder should be inspected to make sure there are no foreign bodies, stones or tumors. The resection plan varies based on surgeon preference but should always be undertaken in a methodical approach. While a variety of resection schemas are acceptable, almost all surgeons recommend resection of the median lobe (if present) as the first step. Resection of the lateral lobes is undertaken either by starting at the anterior or posterior (floor) of the prostate. In either case the resectionist should resect adenoma until the underlying bladder neck fibers or capsule of the prostate are exposed. Resection channel should be carried distally over the length of the prostate and stopped proximal to the verumontanum so that the external sphincter is not injured.
After the initial channel has been created, the resectionist should extend this laterally to “widen” the channel and complete the resection of the lobe. Once the length of the resection has been established, the surgeon should strive to taken long smooth cuts that break the prostate down into “canoe-like” chips. A synchronized rocking motion of the scope will help to reach the more distant parts of the resection in the lateral lobes of larger glands. Once one lobe is complete, resection of the contralateral lobe can be undertaken.
The pace of passage of the cutting electrode through the tissue is important, particularly with the bipolar system, as it allows for ongoing coagulation during the procedure. While the resectionist is concerned with char and over-cauterization of TURBT specimens, this is not a consideration during TURP. Comparatively, the pace of electrode movement TURP should be more controlled as it will improve coagulation and decrease operating room times by minimizing extra measures to control bleeding.
Intraoperative complications and management
Intraoperative bleeding during TURP is common and hemostasis should be maintained through the procedure as an incremental process. If not controlled during the procedure, the sheer volume of small bleeding areas can become overwhelming to the surgeon. A recent analysis of RCTs found that 4.4% of patients required transfusion after TURP (6), emphasizing the importance of maintaining hemostasis.
Arterial bleeding is characterized by a bright red, pulsatile appearance. A more advanced resectionist may continue to resect areas of arterial bleeding if not yet to the depth of the surgical capsule. The resectionist may just slow the resection over the areas of arterial bleeding in a hope to coagulate the bleeding area with the next resection swipe. This of course improves efficiency of the overall procedure but increases the potential for overwhelming bleeding. Above all else, the resectionist should not move the resection away from a bleeding and exposed artery until it has been controlled. Often arterial bleeding is not completely controlled until the capsule is reached in that area. During the operation, the resecting loop should be placed against the bleeding vessel to tamponade the vessel with the fulguration pedal then pressed as it allows for more selective (and effective) vaporization. Haphazard coagulation should be discouraged is it can lead to delayed hemorrhage due to sloughing of tissue.
Venous bleeding is common during TURP and is more characterized by bleeding that appears to be occurring “off screen” or is dark in color. While viewing a concerning area, venous bleeding may be temporarily stopped either by the hydrostatic pressure in the bladder and prostate fossa from a full bladder or due to the irrigant running over the area and obscuring the area of bleeding. Bleeding at the bladder neck (particularly anteriorly) can be facilitated by reducing bladder volumes to allow these parts of the prostate to be better visualized.
Hemostasis should be confirmed at the end of the case with the resectoscope parked at the apex, the bladder minimally distended, and inflow off. Evasive persistent arterial bleeding usually has an anterior location thus if the resectionist cannot locate the source continued bleeding one would be wise to concentrate the investigation in that location. If venous bleeding cannot be completely controlled, as is frequently the case, the catheter can be connected to continuous bladder irrigation (CBI) and/or traction to maintain pressure on the prostatic fossa. If applying traction, a large bore catheter is inserted, and the balloon inflated with 20 cc more than the resected weight. “Orthopedic traction” can be applied by tying the catheter to a roll of narrow gauze or umbilical tape on the proximal end and a 250–500 cc bag of fluid that hangs over the foot of the bed on the distal end. Alternatively, traction can also be applied by using adhesive tape to attach the catheter to the proximal thigh, although it is our experience that orthopedic traction is more effective.
Undermining of the bladder trigone by over-resection of the proximal prostatic urethra is problematic for a variety of reasons. Catheter placement both postoperatively and in the case of a failed voiding trial may be difficult. Many surgeons will resect the 6 o’clock portion of the prostate at the end of the case as frequent passes of the resectoscope across the prostate-vesical junction can cause repeated injury and worsening of the over-resection and lead to disruption of the junction. Foley catheter should be placed using a guidewire or catheter guide and the surgeon should consider delayed catheter removal if the patient is high risk to fail voiding trial. An intraperitoneal perforation is unlikely but possible with this complication and can be ruled out with cystogram.
Capsular perforation can lead to venous bleeding as venous sinusoids are opened. Symptomatic perforations are rare and fluid extravasation is customarily extraperitoneal and clinically insignificant. Bleeding from capsular perforations can be difficult to control however and aggressive coagulation of venous sinusoids should be avoided as it may aggravate capsular perforation. Conservative management with catheter drainage may be sufficient for even large intraperitoneal perforations unless the patient is hemodynamically unstable or clinically symptomatic (7-11). For problematic collections, percutaneous aspiration or drain placement will likely be sufficient.
UO injury is an infrequent complication of TURP and is likely more common with a large median lobe which obstructs visualization. The risk can be decreased by resecting the median lobe just in the midline until enough of the median lobe is removed that the UOs can be visualized. If cutting current was used during an errant portion of resection, a ureteral stent is usually not needed. If coagulating current was used, a ureteral stent should be considered and removed 4 to 6 weeks later to allow the UO to heal without stricture. Ureteral patency should be ensured by IV pyelogram, retrograde pyelogram or inferred by renal ultrasound. Delayed imaging should also be considered 2–3 months later to exclude delayed stricture.
During a TURP the internal urethral sphincter, which is the predominant continence mechanism in men, is resected and rendered incompetent (12). It is vital that the surgeon preserve the external urethral sphincter to prevent total or stress urinary incontinence. The verumontanum is an invaluable landmark which should be preserved during TURP and many surgeons will make “char” marks proximal to the veru before starting the resection (or vaporization). The external urethral sphincter is a complex of smooth and striated muscle fibers that is not always clearly demarcated, so excessive resection or fulguration near the verumontanum should be avoided. Up to 5% of patients report postoperative stress urinary incontinence, although this is typically transient as long as the external urethral sphincter has been preserved (13).
Before terminating the procedure, the prostate chips in the bladder should be removed with careful attention paid to make sure any bladder diverticula are also empty. The resected area should be carefully examined for any residual bleeding with the bladder relatively empty so that hydrostatic pressure is not unknowingly and temporarily stopping any venous bleeding. A large bore catheter should be placed into the bladder. The amount of fluid placed in the balloon should be tailored to the volume of resected tissue to avoid the catheter balloon falling into the excavated prostatic fossa. In our practice, the Foley catheter is generally placed to traction for a short time with release of traction based on residual hematuria. The use of CBI is not compulsory but can be used if the irrigant is not clear at the end of the case but will require a three-way catheter.
Postoperative complications and management
Improvement of technology and refinement of technique has reduced the complication rates of TURP, with contemporary studies demonstrating a 5% risk of reoperation within 5 years and 1–10% complication rate for TURP performed for BPH (14-19). Increasing gland size and pre-operative retention appear to be risk factors for additional procedures (20).
Bladder neck contracture (BNC) rates have been fairly consistent over time at around 2% (13,21) but have occasionally been quoted at much higher rates. Over-fulguration of the bladder neck during the procedure is thought to be causative and patients will generally report worsening urinary flow rates around 6 months from TURP (21). Office cystoscopy is diagnostic and gentle dilation using sounds or balloon can be tried as first line. After more conservative approaches have failed, endoscopic incision in the OR is generally the next step. We recommend more aggressive incision at this time with the expectation of peri-vesical fat in the lateral incisions.
The larger scope required during TURP may predispose to urethral stricture and a rate of 4.1% was found in an analysis of 34 randomized controlled trials (6). The scope should be kept well lubricated and movement of the scope within the urethra should be minimized to decrease rates of this complication.
PVP
Introduction
The contemporary laser used for PVP is derived from a variety of laser technologies. The potassium-titanyl-phosphate (KTP) and lithium-triborate (LBO) lasers were derived from the 1,064 nm wavelength Nd:YAG. The beam is passed through a KTP or LBO crystal that decreases the wavelength to 532 nm while doubling the frequency. This is a desired wavelength as it is preferentially absorbed by hemoglobin. Use of energy at this wavelength allows for light to move freely though the irrigating fluid and interact with tissue in a way that primarily vaporizes tissue with minimal but effective coagulation.
Technical factors
The fiber is side-firing with the energy emitted at 70° to the fiber’s longitudinal axis. The fiber is introduced through a scope wherein the aiming beam can be seen and focused on the target area on the prostate. When activated, the energy is then used to vaporize the prostate in an “inside to out” fashion. Vaporization terminates when fibers from the surgical capsule are visualized.
The modern fiber now includes a separate water supply that independently cools the fiber. Previous versions have relied on the irrigant used during the case to work as a heat sink to maintain the fiber temperature and previous versions of the fiber were prone to overheating especially when working in a restricted space. Before starting the surgeon should ensure that the accessory water supply for the fiber is freely flowing. The fiber may also overheat and wear excessively if tissue becomes attached to the glass oculus from which the laser energy is emitted. This area should be kept clean of tissue during the case and may be wiped down with a damp sponge if it becomes occluded.
The power selected, distance from the laser fiber to the prostate tissue (working distance), sweep speed and sweep angle are important factors to consider during the procedure. The laser energy interacts with the tissue and local thermal energy is created. If this thermal load is below the boiling point of the tissue, then tissue coagulation will occur often with delayed tissue death and sloughing. If the energy is above the boiling (or vaporizing) temperature for the tissue then vaporization will occur. Vaporization is more efficient with regards to tissue removal and will minimize postoperative irritative urinary complaints.
To optimize outcomes and preferentially achieve vaporization over coagulation, the surgeon should monitor the power used during the procedure and the distance between the laser fiber and prostate parenchyma (working distance). If the working distance is too short (fiber too close to tissue) then contact vaporization may occur with resulting damage to the fiber tip. However inefficient energy use and increased tissue coagulation will occur if the working distance is too large. Maintaining an optimal distance throughout the procedure will minimize tissue damage improve patient outcomes. We strive for a working distance of about 3 mm between the fiber tip and the tissue.
The laser power should be monitored throughout the case and adjusted based on tissue factors and proximity to important anatomical landmarks. Lower power settings are recommended around the verumontanum/prostate apex and the bladder neck to minimize injury to the sphincter and bladder, respectively. Once away from these important structures the surgeon should use higher power settings to minimize operating room times and maximize tissue vaporization. The production of large bubbles during energy use is indicative of vaporization and efficient energy use.
The sweep speed (rotational angular speed speed) and sweep angle are other important technical factors to consider during the procedure. A smooth and continuous sweeping motion is vital to minimize irregularities in the depth of vaporization. The sweep angle and speed are important to consider to optimize vaporization. Research from an in vitro study found vaporization was optimized when the angle was between 15 and 30 degrees with a minimization of coagulation at 30 degrees of sweep (22). Larger sweep angles (greater than 45 degrees) allowed for wider but more superficial vaporization defects (23) which will hamper efficiency. We agree that a sweep speed of roughly 2 seconds per each 30 degrees rotational angle is reasonable (24) however we generally advocate for a slightly faster sweep speed. Novices, or those instructing novices, will notice that they generally have quicker sweep speeds over less of a sweep angle. We routinely encourage methodical, controlled movements and to “let the laser do the work”.
Intraoperative plan
A similar survey of the anatomy and instruments should be undertaken as described in the TURP section. Starting at the bladder neck is generally preferred as it removes the more proximal portion of the prostate and allows for free flow of irrigant in case of later bleeding. The bladder neck is generally vaporized until it is level with the trigone of the bladder. The surgeon may choose to do this with either a single midline vaporization or with two channels at the 5 and 7 o’clock position. Generally, we choose to take the two channel approach especially if there is a prominent median lobe as it allows the protruding portion to be “teed up” for future vaporization. The vaporization of the median lobe is then done at a more horizontal angle that would not cause harm to the UOs if the laser is activated more proximal to the prostate plane being vaporized. When making these initial channels the laser should be pointed at an angle off the direct posterior plane so that vaporization can be visualized and any potential rectal injury is minimized by over exuberant vaporization.
Once the bladder neck and any median lobe have been vaporized, the lateral lobes of the prostate should then be vaporized in a sweeping fashion until the fibers of the surgical capsule are visualized. We highly recommend vaporization of this more “safe” portion of the prostate at a high power setting to minimize postoperative storage symptoms and improve efficiency. Once this is complete a lower energy setting can be used to vaporize near the verumontanum to minimize any potential thermal injury to the external urinary sphincter.
Intraoperative complications and management
The safety profile of the PVP technology is excellent. In most studies, urinary outcomes compared to conventional TURP are similar with a decreased risk of peri and postoperative complications (25,26).
Blood transfusions in the peri-operative period around PVP are exceedingly rare even in patients on anti-coagulation or anti-platelet medications (27-29). When compared to M-TURP, PVP had a lower risk (OR =0.10) for perioperative blood transfusion (30). Venous bleeding can be managed generally by continuing vaporization with preferential treatment of the area around the bleeding instead of directly on the bleeding. Venous bleeding will occur in deeper sections of vaporization that are not well visualized. By vaporizing around the area of bleeding, the feeding vessels have been treated which will generally stop the bleeding. In cases where that tactic fails, the surgeon is now better able to visualize the bleeding area and has not created a deeper crevice in the prostate parenchyma. Even when bleeding is pulsatile (and likely arterial) the surgeon should start with the same approach as above. If the bleeding is not able to be controlled, conversion to Bugbee electrode may be helpful to control bleeding (remember to change to a non-ionic irrigant). Conversion to TURP has been reported and can occur either due to inefficient tissue removal or, more commonly, bleeding not able to be controlled with PVP. In some cases before definitely converting to TURP (or possibly while the operating room staff gets the necessary equipment) a trial with a catheter placed on traction to try to stop bleeding may be considered.
Capsular perforation has been reported with PVP and generally is recognized due to increased and focal bleeding in a recently vaporized area. Many surgeons find visualization of the capsule to be more difficult and the risk of perforation is likely increased if there are irregularities in the depth of vaporization. A proper sweeping motion during vaporization and an even removal of tissue limits irregularities in the contour of vaporization and will minimize capsular perforation. If capsular perforation does occur and bleeding cannot be controlled, placement of catheter on traction will generally tamponade bleeding without further issues, however conversion to TURP due to bleeding is more common when capsular perforation occurs (31).
UO injury is a potential but rare complication of PVP. This will generally occur due to errant laser energy use when the UOs are close the bladder neck. We recommend a few strategies to limit this occurrence. While vaporizing the bladder neck, the bladder should be kept relatively full. When this is done the stretch placed on the bladder will “draw” the UOs away from the bladder neck. The angle at which energy is applied to the bladder neck should also be closely monitored and aimed in a way that would not cause UO injury. If channels are created at the 5 and 7 o’clock area during vaporization, we generally use a very lateral facing vaporization so that either UO (ipsilateral or contralateral) is not vaporized by inadvertent medially facing energy. Once the median lobe has been isolated by creating the channels, the fiber is always facing above, or at least at, a horizontal plane when vaporizing the prostate near the bladder neck.
Rectal injury is an uncommon occurrence during PVP and can be minimized by not applying laser energy in a purely posterior direction. The floor or posterior aspect of the prostate can be more difficult to treat than in the conventional TURP. This is due to the angle of the laser fiber emission and the inability to treat tissue directly in front of the fiber that is treatable with the TURP loop. This becomes an increasing problem in larger prostates where a high bladder neck dominates due to the large volume of posterior tissue. When vaporizing this posterior tissue, we apply laser energy once again in a primarily horizontal plane and vaporize “layers” of posterior tissue from superficial to deeper aspects of the parenchyma. By never pointing directly posterior any overly zealous vaporization will not injure the rectum.
Postoperative complications and management
Dysuria and storage symptoms are commonly reported after any BOO procedure. Generally, the degree of dysuria after PVP is thought to correlate with the volume of coagulated tissue (32). Other patient factors that have been postulate to predispose to post-operative irritative symptoms include having a large median lobe, previous prostatitis, dense/fibrous prostate tissue and certain previous prostate procedures (TUNA, TUMT) that reduce prostate vascularity. The use of preoperative finasteride and patients with lower pre-procedure AUASS have been shown to be risk factors (33). The appropriate use of higher laser power during the case is preventative of these symptoms in our experience. While these symptoms are generally self-limited and transient, the physician should have an armamentarium of options for these unhappy patients.
Reassurance of the generally self-limited nature of these issues should be the first line for the physician although problems like a UTI or retained fiber fragment should be considered. A urinalysis will generally demonstrate a sterile pyuria. Pharmacologic interventions can help bridge the patient until the “tincture of time” can work. These interventions should be tailored to the patient’s symptoms. Patients with dysuria will more likely respond to a short course of pyridium if symptoms are mild. In more significant or persistent symptoms, a trial of NSAIDs or a short course (5–7 days) of a steroid taper will reduce post-operative inflammation. If urinary storage symptoms predominate, the physician can prescribe a few months of an anti-cholinergic although we find most patients do not want to add additional medication when they are reassured these irritative symptoms are temporary.
Generally, incontinence post-procedure is urge related. However, the physician should carefully rule out stress related causes that may be due to sphincteric injury. Once again, anticholinergics can be offered to patients with urge related postoperative incontinence. This finding is also generally self-limited but can take 3–6 months to resolve and the patient should be reassured as such.
Conclusions
With an increasing number of available surgical options, surgeons performing endoscopic techniques for LUTS/BPH should be well acquainted with the variety of treatment options for men who have failed medical management. TURP and PVP remain the mainstay of treatment of LUTS/BPH, therefore urologists should be familiarized with surgical techniques, complications, and results. A better understanding of these technologies and the management of their complications will help to maximize patient outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lee E, Yoo KY, Kim Y, et al. Prevalence of lower urinary tract symptoms in Korean men in a community-based study. Eur Urol 1998;33:17-21. [Crossref] [PubMed]
- Norman RW, Nickel JC, Fish D, et al. 'Prostate-related symptoms’ in Canadian men 50 years of age or older: prevalence and relationships among symptoms. Br J Urol 1994;74:542-50. [Crossref] [PubMed]
- Berges RR, Pientka L. Management of the BPH syndrome in Germany: who is treated and how? Eur Urol 1999;36 Suppl 3:21-7. [Crossref] [PubMed]
- Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet 1991;338:469-71. [Crossref] [PubMed]
- Issa MM. Technological advances in transurethral resection of the prostate: bipolar versus monopolar TURP. J Endourol 2008;22:1587-95. [Crossref] [PubMed]
- Mayer EK, Kroeze SG, Chopra S, et al. Examining the “gold standard”: a comparative critical analysis of three consecutive decades of monopolar transurethral resection of the prostate (TURP) outcomes. BJU Int 2012;110:1595-601. [Crossref] [PubMed]
- Park SK, Cho WJ, Choi YS. Fluid extravasation caused by bladder perforation during bipolar transurethral resection using saline solution -a case report. Korean J Anesthesiol 2013;65:163-6. [Crossref] [PubMed]
- Mamoulakis C, Trompetter M, de la Rosette J. Bipolar transurethral resection of the prostate: the 'golden standard' reclaims its leading position. Curr Opin Urol 2009;19:26-32. [Crossref] [PubMed]
- May F, Schlenker B, Hofer B, et al. Laparoscopic repair of iatrogenic bladder perforation during transurethral bladder tumor resection: Case report and literature review. Indian J Urol 2013;29:61-3. [Crossref] [PubMed]
- Bishay M. John D'A Honey R. A minimally invasive approach to transperitoneal perforation of the bladder during bladder tumour resection. Can Urol Assoc J 2016;10:E117-8. [Crossref] [PubMed]
- Pansadoro A, Franco G, Laurenti C, et al. Conservative treatment of intraperitoneal bladder perforation during transurethral resection of bladder tumor. Urology 2002;60:682-4. [Crossref] [PubMed]
- Rolnick HC, Arnheim FK. An anatomic study of the external urethral sphincter in relation to prostatic surgery. J Urol 1949;61:591-603. [Crossref] [PubMed]
- Ahyai SA, Gilling P, Kaplan SA, et al. Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. Eur Urol 2010;58:384-97. [Crossref] [PubMed]
- Bhojani N, Gandaglia G, Sood A, et al. Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. J Endourol 2014;28:831-40. [Crossref] [PubMed]
- Borboroglu PG, Kane CJ, Ward JF, et al. Immediate and postoperative complications of transurethral prostatectomy in the 1990s. J Urol 1999;162:1307-10. [Crossref] [PubMed]
- Hong JY, Yang SC, Ahn S, et al. Preoperative comorbidities and relationship of comorbidities with postoperative complications in patients undergoing transurethral prostate resection. J Urol 2011;185:1374-8. [Crossref] [PubMed]
- Horninger W, Unterlechner H, Strasser H, et al. Transurethral prostatectomy: mortality and morbidity. Prostate 1996;28:195-200. [Crossref] [PubMed]
- Rassweiler J, Teber D, Kuntz R, et al. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol 2006;50:969-79; discussion 80. [Crossref] [PubMed]
- Varkarakis J, Bartsch G, Horninger W. Long-term morbidity and mortality of transurethral prostatectomy: a 10-year follow-up. Prostate 2004;58:248-51. [Crossref] [PubMed]
- Reich O, Gratzke C, Bachmann A, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol 2008;180:246-9. [Crossref] [PubMed]
- Greene L, Holcomb G. Transurethral resection in special situations. Transurethral surgery Philadelphia: WB Saunders 1979:216.
- Ko WJ, Choi BB, Kang HW, et al. Defining optimal laser-fiber sweeping angle for effective tissue vaporization using 180 W 532 nm lithium triborate laser. J Endourol 2012;26:313-7. [Crossref] [PubMed]
- Osterberg EC, Kauffman EC, Kang HW, et al. Optimal laser fiber rotational movement during photoselective vaporization of the prostate in a bovine ex-vivo animal model. J Endourol 2011;25:1209-15. [Crossref] [PubMed]
- Zorn KC, Liberman D. GreenLight 180W XPS photovaporization of the prostate: how I do it. Can J Urol 2011;18:5918-26. [PubMed]
- Bachmann A, Tubaro A, Barber N, et al. 180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial--the GOLIATH study. Eur Urol 2014;65:931-42. [Crossref] [PubMed]
- Bachmann A, Tubaro A, Barber N, et al. A European multicenter randomized noninferiority trial comparing 180 W GreenLight XPS laser vaporization and transurethral resection of the prostate for the treatment of benign prostatic obstruction: 12-month results of the GOLIATH study. J Urol 2015;193:570-8. [Crossref] [PubMed]
- Ruszat R, Wyler S, Forster T, et al. Safety and effectiveness of photoselective vaporization of the prostate (PVP) in patients on ongoing oral anticoagulation. Eur Urol 2007;51:1031-8; discussion 8-41. [Crossref] [PubMed]
- Woo HH, Hossack TA. Photoselective vaporization of the prostate with the 120-W lithium triborate laser in men taking coumadin. Urology 2011;78:142-5. [Crossref] [PubMed]
- Sandhu JS, Ng CK, Gonzalez RR, et al. Photoselective laser vaporization prostatectomy in men receiving anticoagulants. J Endourol 2005;19:1196-8. [Crossref] [PubMed]
- Cornu JN, Ahyai S, Bachmann A, et al. A Systematic Review and Meta-analysis of Functional Outcomes and Complications Following Transurethral Procedures for Lower Urinary Tract Symptoms Resulting from Benign Prostatic Obstruction: An Update. Eur Urol 2015;67:1066-96. [Crossref] [PubMed]
- Bachmann A, Muir GH, Collins EJ, et al. 180-W XPS GreenLight laser therapy for benign prostate hyperplasia: early safety, efficacy, and perioperative outcome after 201 procedures. European urology 2012;61:600-7. [Crossref] [PubMed]
- Choi B, Tabatabaei S, Bachmann A, et al. GreenLight HPS 120-W laser for benign prostatic hyperplasia: comparative complications and technical recommendations. Eur Urol Suppl 2008;7:384-92. [Crossref]
- Matoka DJ, Averch TD. Predictability of irritative voiding symptoms following photoselective laser vaporization of the prostate. Can J Urol 2007;14:3710-4. [PubMed]