Vasectomy reversal: decision making and technical innovations
Introduction
Vasectomy is the contraception method of choice for more than 500,000 American men annually, and upwards of 8% of married couples worldwide (1,2). Although patients should be counseled to consider vasectomy a permanent means of contraception, close to 20% of men express a desire for children after the procedure (3). For men who want to achieve natural conception after vasectomy, vasectomy reversal (VR) represents the only option. Approximately 2–6% of American men will ultimately undergo reversal (1,3).
VR is a microsurgical procedure performed as either a vasovasostomy (VV) or epididymovasostomy (EV). These procedures were first described in the early 20th century (4). Over the past century, a great deal of effort and research has been dedicated to improving both the technology and the technique of VR (4). This article aims to share the authors’ experiences, drawn from over thirty-five years of practice, to provide insight into the practice of VR, from preoperative counseling through intraoperative techniques and concluding with postoperative care and how to manage difficult situations.
Patient evaluation and counseling
Because pregnancy and live birth are the ultimate goals following a VR, both the male and female must be evaluated before the procedure is recommended.
Female evaluation
Advanced maternal age is associated with decreased fertility potential (5). Unfortunately, there is no clear consensus regarding which women require a dedicated evaluation prior to their partner’s undergoing a VR. The American Society of Reproductive Medicine (ASRM) recommends that women over the age of 35 be offered an expedited fertility evaluation, but it provides no specific age cutoffs for couples specifically pursuing VR (5). A retrospective review of 212 patients undergoing VR showed that female partner age greater than 40 years was an independent predictor of lower pregnancy rates (6). The authors recommend that any nulliparous female partners over the age of 35 be offered consultation with a reproductive endocrinologist so that the value of further testing such as ovarian reserve determination or hysterosalpingography can be discussed.
Male evaluation
The man’s fertility history is important to establish an estimate of his baseline fertility. If he has previously had difficulty initiating a pregnancy, in the absence of any prior identifiable female factor, he likely will continue to have difficulty in the future (7). Attention also should be paid to any history of pelvic surgeries or hernia repairs that could cause an additional vasal obstruction. In such a case, an intraoperative vasogram may be merited. Medical history should also include inquiry into prior use of anabolic steroids or testosterone supplement therapy (TST). TST is a well-known cause of impaired spermatogenesis (8,9). For men reporting utilization of TST within the past 6 months, we perform blood testing to evaluate hormonal status and implement a regimen of testicular salvage therapy consisting of clomiphene citrate and human chorionic gonadotropin (hCG) (10). An in-office testicular aspiration is also considered for any man with a history of TST in order to confirm return of spermatogenesis prior to VR.
Physical examination also is relevant for proper assessment and counseling. Ideally, the exam should be performed in a warm room to allow for easier evaluation of the scrotal contents. After initial evaluation of the cord structures, attention should be directed towards identifying the level of the prior vasectomy site, the length of vasal defect, and the presence of granulomas. If the vasectomy site is low and close to the epididymis, it is more likely that an EV or VV with anastomosis of the convoluted portion of the vas deferens would be needed. A long vasal defect requires that the testicle be brought cranially to close the gap. This is both a technical consideration and a cosmetic one because the testicles may appear postoperatively to sit higher in the scrotum. Granulomas should also be noted as their presence suggests that sperm have been traveling to the distal portions of the vas deferens and thus portend a higher likelihood of the need for a VV. Additionally, the size and density of the testicles should be evaluated since smaller and softer testicles suggest damaged seminiferous tubules and thus decreased sperm production.
Preoperative counseling
Patient counseling should incorporate the findings from the history and physical examination to set appropriate expectations for the postoperative outcome. Obstructive interval (OI) is one of the most important influencing factors. There is debate, however, regarding how much OI impacts patency rates. The VV study group showed a gradual decline in patency rates while Silber found a more specific decline at an OI of 10 years (11,12). A more contemporary study by Magheli et al. conversely reported a relatively consistent patency over the first 10 to 15 years (13). While patency rates may be preserved as OI increases, these outcomes are notably dependent on the surgeon’s ability to perform a high quality EV in addition to a VV, as the likelihood of requiring an EV increases with OI (13,14).
The authors utilize a nomogram as a part of preoperative counseling to predict the likelihood of the patient’s requiring an EV. The preferred nomogram was published by Fenig et al. in 2012 and incorporates patient age, years since vasectomy, vasectomy site, epididymal fullness, and presence or absence of granuloma to preoperatively predict the need for EV (15). This is particularly helpful in setting expectations and provides valuable context for the patient’s consideration of intraoperative sperm extraction as discussed below. This nomogram can be downloaded as a free application “vas reverse” from the app store.
Role of intraoperative sperm extraction and cryopreservation
Sperm extraction and cryopreservation at the time of VR should be discussed preoperatively. We offer this as an option for patients who want to avoid a future sperm extraction in the event of persistent oligospermia or azoospermia following repair. In practice, the value of sperm extraction is fairly low considering the cost of retrieval and high likelihood of patency following most reversals. That being said, this procedure may be appropriate for couples with lower fertility potential due to age or other comorbidities or as an intraoperative choice if only a bilateral EV is required. We perform cryopreservation for approximately 20% of our patients undergoing VR and specifically recommend cryopreservation as an intraoperative decision for men who require a bilateral EV.
Surgical technique
Equipment
The microsurgical set that the authors prefer is available through Accurate Surgical and Scientific Instruments Corporation (ASSI). The set (reference ASSI.LVAS1) includes the various forceps, dissecting scissors, needle holder, nerve holder and blade holder necessary to perform either a VV or EV. Of the two microsurgical sutures currently preferred, 10-0 double armed nylon is used for the luminal stitches during a VV and for the epididymal tubule to vas lumen for an EV. A double-swaged harpoon (bicurve) needle is utilized (Sharpoint®). The 9-0 nylon single armed sutures are preferred for the muscular layer during a VV and for the vasal muscular to epididymal tunic during an EV. Because of the delicate nature of both the equipment and sutures, it is necessary to have duplicate instruments and redundant sutures available.
Anesthetic and patient positioning
General anesthesia is preferred because of the duration of the procedure and the benefits of paralysis during the delicate steps of an EV. For patients refusing general anesthesia, neuraxial anesthesia with sedation is a reasonable alternative. The patient should remain in the standard supine position throughout the operation.
Following induction of anesthesia, the patient’s pubic hair is clipped using electric shears and the patient surrounding area is prepped with a mixture of chlorhexidine gluconate and isopropyl alcohol. The groin and penis are included in the prep to allow for extension of the incisions cephalad if necessary. Waterproof drapes are useful to help keep the patient dry as there is copious use of irrigation throughout the surgery. The operative microscope is positioned on the left side of the head of the operative table and the table is slid to its caudal extent to accommodate the surgeon’s legs while sitting. The surgeon should sit on the side of the patient with his dominant hand towards the foot of the patient.
Operative technique
VV
The procedure is begun by marking bilateral vertical incisions that originate mid-testis and extend approximately 5 cm along the path of the spermatic cords. Starting on the right side, the testis and spermatic cord are delivered, while the tunica vaginalis is kept intact. The testicular end of the vas is then isolated with a towel clamp just proximal (caudal) to the vasectomy site. While caudal traction is placed on the towel clamp, a curved iris scissor is passed posterior to the vas with care taken to avoid coming too close to the vas deferens in order to preserve the blood supply. The assistant then passes a Jacobson mosquito forceps back through this window. The next step is to use this space to further dissect the vas deferens with the goal of preserving the peri-vasal blood supply and creating a space large enough to accommodate the vas holding forceps (nerve clamp) in a tension-free manner. A 5-0 chromic stay suture is then placed through the adventitia of the vas, proximal to the anticipated site of transection.
The vas is next transected utilizing a 2 to 3.5 mm vas-holding forceps to stabilize the vas and a Dennis blade to pass quickly through the slot and cleanly divide the vas. It is helpful to have the assistant stabilize the vas with a Jacobsen forceps in the caudal crotch posterior to the vas and an Adson forceps holding the cephalad vasectomy site. Care is taken to avoid placing the tension on the vas during cutting as this leads to protrusion of mucosa from the vasal lumen. Expected bleeding is then controlled with a micro-tipped bipolar coagulation device, utilizing a power level of 3 around the vas in order to avoid tissue devascularization. The segment of the vas deferens cephalad to the vasectomy site is then isolated and transected in a similar fashion, again taking care to maintain the vasal blood supply. Once the vas has been transected on both sides of the vasectomy site, this intervening segment is excised using cautery with a needle tip.
The next step in the procedure is to evaluate the fluid from the testicular vas in order to determine the type of reversal to be performed. If fluid is not freely flowing from the vas, gentle squeezing of the vasal segment and the epididymis will aid efflux. A 3 mL syringe primed with a small amount of human tubal fluid (HTF) and fitted with a 25 Gauge angio-catheter tip is used to collect a sample of vasal fluid. The fluid is then inspected under a table-top phase microscope at 400× magnification. As discussed in the intraoperative considerations portion of this article, the appearance of the fluid and microscopic findings influences the decision to proceed with VV or EV. If there are motile sperm and the patient desires cryopreservation, it is possible to collect this fluid for such a purpose.
Once the decision is made to proceed with a VV, the ends of the two vasal segments are approximated using a 5-0 PDS passed through the loose adventitia surrounding the vasal ends. A moist sponge may be placed under the testicle to bring the testicle cephalad, and elastic retractor hooks are helpful to retract redundant scrotal skin and surrounding tissue. With use of the pre-placed stay sutures to manipulate the vasal segments, the approximation is evaluated to ensure that the two ends can meet in a tension-free fashion and without overlap.
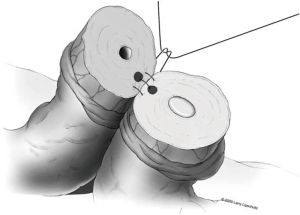
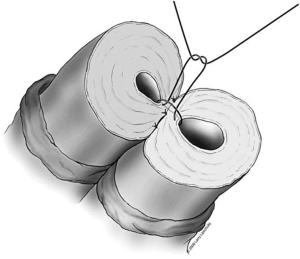
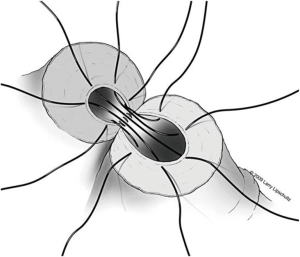
A two-layer technique is the preferred method for anastomosis. This begins with three 9-0 nylon sutures through the muscular layer, the first placed at the 6 o’clock position and then at the 5 and 7 o’clock positions (Figure 1). It is helpful to mark the 6 o’clock positions on each vas with a fine-tipped marking pen prior to the first suture. Once these first three sutures are passed and tied, the mucosal edges should be closely approximated at the 6 o’clock position. Next, interrupted 10-0 sutures are placed through the mucosal layer and tied sequentially at the 6 o’clock, 4 o’clock, and 8 o’clock positions (Figure 2). Throughout this process, a fine jewelers forcep can be used to intermittently dilate the vasal lumen. Methylene blue dropped onto the lumen is also useful to help delineate the mucosal edge. Three to five additional 10-0 nylon sutures are placed circumferentially and left untied until each has been place (Figure 3). It is important to pass the 12 o’clock mucosal suture particularly superficial so as to avoid bunching of the mucosa at this position. Each suture is then tied down to complete the mucosal layer. Starting at the 12 o’clock position, interrupted 9-0 nylon sutures through the muscular layer are then placed and tied circumferentially to complete the anastomosis (Figure 4). If redundant adventitia is mobile and available, a third layer may be approximated to provide additional support and blood supply. At this point, the anastomosis is complete.
EV
If an EV is selected as the reversal approach, the first step is to ligate the end of the testicular vasal segment with a 5-0 chromic suture. The testicle should then be delivered through a linear incision in the tunica vaginalis in order to expose the epididymis. With the non-dominate hand, the surgeon then grasps the epididymis between the thumb and forefinger. The tubules are then inspected with the goal of identifying the dilated tubules proximal to the presumed obstruction. Once identified, the most distal available dilated tubules should be chosen so as to allow dissection of another more proximal site if necessary. Additionally, transit through the epididymis is necessary for sperm development, and choosing a more distal site may maximize this process.
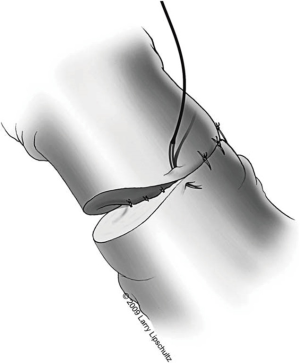
Once a site is chosen, the tunic of the epididymis is opened approximately 0.5 cm with micro-dissecting scissors. Passing one blade of these scissors posterior to the tunic circumferentially around the incision helps to mature the dilated tubules with minimal direct manipulation of the fragile tubules themselves. At no time should the tubules be grasped with a forcep as this could lead to rupture. Should a tubule rupture prematurely at any point during this dissection, a new and more proximal site must be chosen. Methylene blue dropped on the tubules is helpful during this process to demarcate the anatomy. The ideal tubule is one that lies perpendicular to the line of the tunical incision and is close enough to the edge of the tunic to approximate the lumen of the vas to the epididymal tubule once the vasal muscularis is secured to the tunic edge (Figure 5).
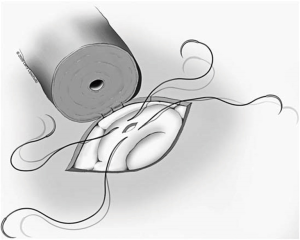
Once a tubule has been chosen, two separated 10-0 nylon double-ended sutures are placed. One of the needles from each suture is placed opposite the other lengthwise along the tubule. The needles are not pulled through but rather left half way through the tubule wall and then rotated laterally to facilitate incision of the tubule between the two needles. An Alcon Surgical® 20 gauge Corneal/Scleral V-Lance Knife is then used to puncture the tubule between the two sutures (Figure 6). The needles are then pulled through the remainder of the tubular wall on each side and placed gently off to the side of the epididymis. The tubule should now have a separate suture in each side of the tubular wall adjacent to the incision in the tubule that will later be intussuscepted into the vasal lumen. Fluid from the tubule should then be aspirated and examined under the table-top microscope. If sperm are seen, the surgeon may proceed with the chosen tubule. If no sperm are seen, a new more proximal site along the epididymis must be chosen and the same procedure repeated. The first tubule is then desiccated with a bipolar cautery.
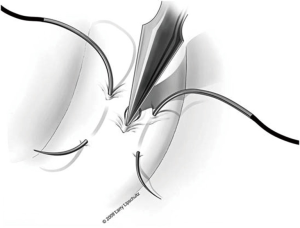
The next step is to approximate the lumen of the abdominal vasal segment to the epididymal tubule. The abdominal vassal segment will likely need to be further mobilized to provide length sufficient to reach the epididymis. The scrotal incision must sometimes be extended cephalad to accommodate this dissection. Again, care must be taken to preserve the adventitia surrounding the vas, which contains critical blood supply. A 5-0 PDS is then passed through the adventitia at the base of the mobilized segment of vas. A window in the tunica vaginalis is created using a Jacobsen hemostat that is then used to pull the 5-0 PDS and vasal segment through this opening. Next, the same 5-0 PDS is used to secure the vasal segment to the mesentery of the epididymis in a location that will allow the vasal lumen to lie in close proximity to the opened tubule (Figure 7). A 7-0 nylon suture is then used to connect the outer muscularis of the vasal segment approximately midway along its length to a corresponding section of epididymal mesentery in order to provide additional support and better position the vas. Three interrupted 9-0 nylon sutures are then sequentially placed through the edge of the epididymal tunic and the muscularis of the vasal segment at 5, 6, and 7 o’clock (Figure 5). These sutures provide an outer strength layer and, once tied, should closely approximate the vasal lumen to the opened tubule.
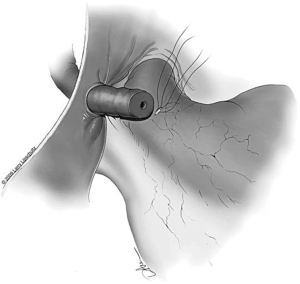
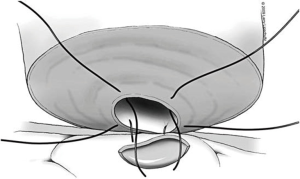
The tubule is now ready to be intussuscepted into the vasal lumen. Using the pre-placed 10-0 sutures, the needles are passed inside out through the sero-muscular layer of the vas in a near-to-near, far-to-far fashion. The two needles on the same side of the luminal opening as the three 9-0 outer layer sutures are passed through the vas at the 4 and 8 o’clock positions (near-to-near). The two 10-0 needles on the other side of the tubular opening are then passed through the vas at the 2 and 10 o’clock positions (far-to-far) (Figure 8). The 10-0 sutures at the 2 and 4 o’clock position are tied in a loose surgeons knot, followed by the 10-0 sutures on the opposite side at the 8 and 10 o’clock position. As these knots are tightened, the tubule should be pulled up and into the lumen of the vas. Additional 9-0 nylon sutures are then used to connect the epididymal tunic to the outer muscular layer of the vas in an interrupted fashion thus completing the outer layer of the anastomosis. The tunica vaginalis is then carefully brought back around the testicle and closed with a running 3-0 chromic suture.
Final steps and closure
Prior to returning the testicle to the scrotum, it is critical to take the necessary time to achieve hemostasis. A postoperative hematoma significantly increases the risk of failure. The authors have had success utilizing the fibrin sealant TISSEEL® to provide further hemostatic control without compromising patency. If there is any concern for persistent bleeding, however minimal, a 7-French closed suction drainage system (TLS Surgical Drainage System® ) may be used to further minimize the risk of postoperative hematoma. The drain is removed 24 hours later. Once the testicle is replaced in the scrotum, the dartos layer is closed with a running 3-0 chromic suture and the skin is closed with a 4-0 fast-absorbing chromic running horizontal mattress stitch.
Intraoperative decision making and challenging situations
Choosing between a VV and EV
The decision to perform a VV or an EV should be based on both the macroscopic and microscopic appearance of the fluid expressed from the testicular vasal segment. Clear fluid portends better patency and pregnancy rates while thick, pasty fluid is associated with worse outcomes (11,16). Microscopically, the presence of whole sperm predicts a better outcome than the appearance of sperm heads only or no sperm at all (17). Historically, the Silber scale has been used to evaluate the microscopic quality of the sample to guide the choice between VV and EV (11,12). On the basis of recent work from Smith et al. which showed greater than 90% patency rates in men with a sample demonstrating sperm heads only and/or short tails, regardless of macroscopic fluid quality, the authors now put less emphasis on this variable and will perform a VV in any scenario in which sperm parts are seen (18). An EV is reserved for those cases in which neither whole sperm nor sperm parts are identified.
There are also circumstances in which fluid from the testicular vas initially shows no sperm but the quality of the sample changes over a short period of time. This is likely due to an accumulation of material near the vasectomy site that must clear in order for more proximal sperm-containing fluid to be expressed. If no sperm are seen on the initial side, the authors will often explore the contralateral side and then re-sample the first side after some time has passed. Not infrequently, this has yielded sperm-containing fluid and allowed for a VV.
Low vasectomy site
If the vasectomy site is in close proximity to the epididymis, it may be necessary to use the convoluted portion of the vas deferens for the testicular side of the VV. This can make the procedure more difficult because of the difficulty in creating a perpendicular cut across the lumen of this more serpentine section of vas. Additionally, the seromuscular layer of the vas is thinner in the convoluted section, and this can make a two-layer closure more difficult. The authors recommend carefully dissecting some of the adventitia surrounding the convoluted tubule to facilitate straightening a short segment to allow the necessary transverse division. Restraint in this dissection is necessary, however, since this layer carries some of the blood supply to the vasal segment. While technically more challenging, using the convoluted vas for a VV has been shown to provide patency rates comparable to those seen with a traditional VV (19).
Vasal obstruction
If the patient has any history of pelvic trauma or inguinal surgeries, it is wise to check for patency of the abdominal segment of vas during the procedure. This can be done by passing a 2-0 prolene suture through the abdominal segment or by instilling methylene blue through the abdominal vas and then catheterizing the bladder to see if any blue contrast reached the urethra. If there is an obstruction, the location can be approximated by measuring the length of Prolene suture that can be passed or by performing an intraoperative vasogram with contrast if fluoroscopy is available. If an obstruction is established in the context of previous hernia repair, the incision can be extended to further dissect the abdominal vas and potentially re-route the vas if the hernia repair is indeed the issue. Another approach would be to perform a trans-VV if the contralateral testicular segment showed no sperm. By tunneling the vas through the midline scrotal Dartos fascia and performing a VV to the contralateral abdominal vas, the surgeon can avoid having to perform an EV. If an obstruction is identified and the approaches described above are unsuccessful or inappropriate, an EV or VV needn’t be performed on that side.
Postoperative care
Scrotal support and mild wound pressure are accomplished with an unrolled Kerlix® gauze, and a jock strap is used for 24 hours postoperatively. Intermittent application of an ice pack is also useful during the first 24 hours to help limit inflammation and swelling. Scrotal support with a jock strap or briefs is recommended for the first two weeks, and sexual activity should be avoided during this same time period. Strenuous activity and heavy lifting should be avoided for the first postoperative month. Oral narcotics are used for pain control, and non-steroidal anti-inflammatories are avoided due to increased, albeit mild, bleeding risk.
Follow-up and ongoing management
The patient returns at 6–8 weeks for a semen analysis (SA) and again for repeat semen analyses every three months until initiation of a pregnancy or at least stabilization of semen quality. If the patient has no sperm on either the initial or subsequent SAs, a 1-month steroid taper with a concurrent non-steroidal anti-inflammatory is prescribed. For patients who demonstrate azoospermia despite initially having sperm in the ejaculate postoperatively, steroid tapers have successfully improved the presumed strictures and resulted in the return of sperm to the ejaculate.
Complications
Although rare, scrotal hematomas should be treated promptly. Surgical evacuation with placement of drains is merited to help avoid breakdown or stricturing of the anastomoses. A 1-month steroid taper is utilized in these patients to help mitigate the increased risk of stricture. Postoperative infections are rare but should be evaluated with scrotal ultrasound to evaluate for abscess. In addition to antibiotics, a one-month steroid taper could be considered in these patients.
Conclusions
VR is a complex microsurgical procedure that has evolved significantly over the past century. Careful preoperative evaluation is necessary to counsel the patient appropriately and to maximize his chances for success. Intraoperative decision-making and surgical technique are additionally critical, as is the management of postoperative complications. It is hoped that this detailed description of operative and perioperative procedures will help improve the success rates among surgeons performing VRs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Barone MA, Hutchinson PL, Johnson CH, et al. Vasectomy in the united states, 2002. J Urol 2006;176:232-6; discussion 236. [Crossref] [PubMed]
- Pile JM, Barone MA. Demographics of vasectomy--USA and international. Urol Clin North Am 2009;36:295-305. [Crossref] [PubMed]
- Sharma V, Le BV, Sheth KR, et al. Vasectomy demographics and postvasectomy desire for future children: Results from a contemporary national survey. Fertil Steril 2013;99:1880-5. [Crossref] [PubMed]
- Kim HH, Goldstein M. History of vasectomy reversal. Urol Clin North Am 2009;36:359-73. [Crossref] [PubMed]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. committee opinion no. 589. Fertil Steril 2014;101:633-4. [Crossref] [PubMed]
- Hinz S, Rais-Bahrami S, Kempkensteffen C, et al. Fertility rates following vasectomy reversal: Importance of age of the female partner. Urol Int 2008;81:416-20. [Crossref] [PubMed]
- Goldstein M. Vasectomy reversal. Compr Ther 1993;19:37-41. [PubMed]
- Kohn TP, Louis MR, Pickett SM, et al. Age and duration of testosterone therapy predict time to return of sperm count after human chorionic gonadotropin therapy. Fertil Steril 2017;107:351-7.e1. [Crossref] [PubMed]
- MacIndoe JH, Perry PJ, Yates WR, et al. Testosterone suppression of the HPT axis. J Investig Med 1997;45:441-7. [PubMed]
- Coward RM, Mata DA, Smith RP, et al. Vasectomy reversal outcomes in men previously on testosterone supplementation therapy. Urology 2014;84:1335-40. [Crossref] [PubMed]
- Belker AM, Thomas AJ Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the vasovasostomy study group. J Urol 1991;145:505-11. [Crossref] [PubMed]
- Silber SJ. Pregnancy after vasovasostomy for vasectomy reversal: A study of factors affecting long-term return of fertility in 282 patients followed for 10 years. Hum Reprod 1989;4:318-22. [Crossref] [PubMed]
- Magheli A, Rais-Bahrami S, Kempkensteffen C, et al. Impact of obstructive interval and sperm granuloma on patency and pregnancy after vasectomy reversal. Int J Androl 2010;33:730-5. [Crossref] [PubMed]
- Chawla A, O'Brien J, Lisi M, et al. Should all urologists performing vasectomy reversals be able to perform vasoepididymostomies if required? J Urol 2004;172:1048-50. [Crossref] [PubMed]
- Fenig DM, Kattan MW, Mills JN, et al. Nomogram to preoperatively predict the probability of requiring epididymovasostomy during vasectomy reversal. J Urol 2012;187:215-8. [Crossref] [PubMed]
- Potts JM, Pasqualotto FF, Nelson D, et al. Patient characteristics associated with vasectomy reversal. J Urol 1999;161:1835-9. [Crossref] [PubMed]
- Scovell JM, Mata DA, Ramasamy R, et al. Association between the presence of sperm in the vasal fluid during vasectomy reversal and postoperative patency: A systematic review and meta-analysis. Urology 2015;85:809-13. [Crossref] [PubMed]
- Smith RP, Khanna A, Kovac JR, et al. The significance of sperm heads and tails within the vasal fluid during vasectomy reversal. Indian J Urol 2014;30:164-8. [Crossref] [PubMed]
- Sandlow JI, Kolettis PN. Vasovasostomy in the convoluted vas deferens: Indications and outcomes. J Urol 2005;173:540-2. [Crossref] [PubMed]


