Avoiding complications: surgery for ischemic priapism
Introduction
Ischemic, or low-flow, priapism is among the most common and challenging urologic emergencies. Management of ischemic priapism may become perplexing if the condition does not resolve after initial treatment attempts, requiring increasingly invasive interventions associated with increasing levels of risk. The goal of this commentary is to condense a career of experience (TF Lue) in the management of ischemic priapism into a concise, practical clinical tool for the reader, specifically physicians who provide urgent care coverage for urology patients. We will describe our current algorithm for the treatment of ischemic priapism in addition to detailing how we arrived at these recommendations. We will also describe why we believe that the presented approach is the best available approach and why we have turned away from alternative methods.
Most importantly, our approach is founded in three basic objectives:
- To obtain rapid and complete resolution utilizing the least invasive intervention possible;
- To minimize the risk of complications from both the condition itself and the treatment for the condition;
- To maximize patient outcomes.
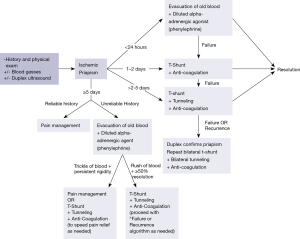
Peer-reviewed, best-practice guidelines for the treatment of ischemic priapism exist (1,2), but randomized controlled trials will likely never be accomplished as priapism is an emergency condition and accrual would be unfeasible. The American Urological Association (AUA) guidelines, first published in 2003 and validated in 2010, are grounded in the best available scientific and clinical evidence combined with expert opinion based on experience (1). The European Association of Urology (EAU) guidelines, first published in 2014, are based on systematic literature review for the best available evidence (2). These documents are informative and useful, but both omit one of the key recommendations of the current commentary: periprocedural anticoagulation (Figure 1). We hope that this recommendation will be strongly considered for incorporation into the next iteration of the guidelines.

Prolonged ischemic priapism produces corporal hypoxia, acidosis, and eventually tissue ischemia. Immediate intervention is required to prevent necrosis and permanent impairment or loss of erectile function. Often, patient factors including embarrassment, uncertainty, or intoxication lead to a delay in the initial presentation for treatment. This delay may be compounded by delays in emergency department triage, assessment, and specialty consultation. Once the diagnosis of ischemic priapism is established, trials of oral agents such as terbutaline or pseudoephedrine, saline irrigation and aspiration, and/or repeated injections of alpha-adrenergic agents may delay definitive management even further. In addition, ischemic priapism may recur after intervention, requiring escalating interventions or transfer to an academic institution, to ensure complete resolution. This scenario is extraordinarily frustrating for the urologist and potentially debilitating for the patient.
It is important to remember that all aspects of sexual medicine are litigious. The urologist should be prepared to have a comprehensive and realistic discussion of the prognosis and treatment options with the patient and his family, with witnesses present if possible. Meticulous documentation of both the counseling discussion and the procedures performed must be accomplished in all cases to protect the urologist if the outcome is poor.
Duration-directed treatment algorithm
Ideally, if prompt resolution of ischemic priapism is accomplished, patient outcomes will improve. We believe that a duration-directed treatment algorithm is the most prudent, physiologic, and straightforward approach to this urgent condition (Figure 2) (3). If such a protocol is followed, the urologist can minimize the risk of treatment complications and maximize patient outcomes. Most cases of ischemic priapism of less than 24-hour duration respond well to aspiration of old blood (not more than 100 mL) from the corpora cavernosa and injection of diluted alpha-adrenergic agents, with the alpha-1 selective phenylephrine (at a dose of 250–500 μg every 3–5 min for up to 30 minutes) being the preferred agent because of its lack of beta-adrenergic cardiac effects. For patients with a duration of less than 48 hours, the majority can be treated with a standard distal T-shunt procedure with pre- and post-operative anticoagulation (Figure 1) being a critical component of the intervention (4). In those patients with a duration over 48 hours, anticoagulation and T-shunt plus intracorporal tunneling (5,6) is effective in the vast majority of patients.
In some cases of refractory priapism, the patient may need to have repeated procedures performed to ensure complete resolution. Prior to repeated intervention, a duplex color ultrasound should be performed to ensure that the patient has priapism and not post-ischemic hyperemia, which is commonly encountered after prolonged periods of ischemia and which may mimic incomplete resolution of priapism. If the patient has post-ischemic hyperemia, duplex color ultrasound will reveal high flow in the corporal arteries. If the patient has recurrent priapism, there will be no flow in the corporal arteries. If ultrasound is not available, penile blood gas may be assessed, but this method is not reliable because even with complete venous flow blockage the oxygen tension within the corpus cavernosa may take 6–8 hours to decrease.
Extraordinary circumstances may lead to an extremely delayed patient presentation of five or more days. If no attempt was made during the 5-day (or longer) interval to re-establish penile blood flow, the chance of penile tissue survival is small, and conservative management with pain medication is an option. However, the provided history may be unreliable or inaccurate, especially in psychiatric patients or intoxicated patients, and there is a possibility that the priapism may have been “incomplete”, allowing for intermittent arterial flow into the corpora. Even after a such prolonged time period, an attempt at needle aspiration and phenylephrine injection should be made if the history is questionable. If ischemic blood rushes quickly out of the corpora and the erection resolves by 50% or more, T-shunt plus tunneling with peri-procedural anticoagulation should be recommended to allow for the best possible outcome. If the blood trickles slowly out and the penis remains rigid despite aspiration and phenylephrine injection, there is little hope that the patient will regain erectile function. In this scenario, pain management becomes the goal of therapy as the erectile tissue is not viable. The two treatment options are pain management alone or T-shunt plus tunneling (with periprocedural anticoagulation) which may speed resolution of pain. If the provided patient history is reliable and the rigid priapism persisted beyond 5 days, pain management alone is the best option as shunting surgery will not change the patient’s outcome.
Thorough documentation of the pre-procedural discussion (anticipated outcomes, options, risks, and benefits) with written informed consent followed by detailed procedural findings after aspiration and injection are crucial in patients with prolonged ischemic priapism. Documentation is particularly important if the patient chooses for pain management alone. If the patient is unable to provide informed consent, following the above algorithm for a patient with an unreliable history is the preferred treatment pathway.
Medical management: aspiration and injection of alpha-adrenergic agent
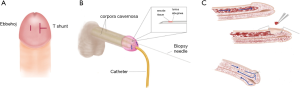
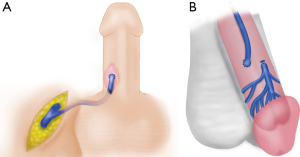
Aspiration or passive drainage of ischemic blood followed by injection with diluted alpha-adrenergic agent should be performed though a butterfly needle of 21 gauge or larger inserted into the lateral aspect of the corpora cavernosum (Figure 3). It is not uncommon for more junior staff members to aspirate excessive amounts of blood during the aspiration process in hopes of achieving improved results. It is important to remind students and residents both that aspiration of 100 mL of blood is sufficient to remove acidotic blood and to facilitate the return of oxygenated blood. Aspiration and injection is successful for 90% of ischemic priapism cases of less than 24 hours duration with complete recovery of baseline erectile capability.
Additionally, when performing injection with alpha-adrenergic agents, it is prudent to ensure that the patient has been thoroughly informed of the details of the procedure and that written informed consent has been obtained. At the same time, it is judicious to obtain approval for shunting procedures at the same setting in the event that the alpha-adrenergic agent injection fails to resolve the priapism. During the procedure of injection, ensure that the patient’s blood pressure and heart rate are monitored with telemetry to examine for changes, such as hypertension, reflex bradycardia, tachycardia, or arrhythmia, associated with intravascular injection of alpha-adrenergic agents. This is particularly important in patients with serious cardiovascular disease. Patients may report headache if the medication reaches the systemic circulation.
If the duration of the priapism episode is over 48 hours, tissue edema from absence of intracorporal circulation and the inability of the damaged cavernous smooth muscle to respond to the medication make intracavernous injection of alpha-adrenergic agents futile. It is understood that most practitioners would nonetheless attempt aspiration and irrigation as this is the least invasive intervention with the lowest associated procedural risk. However, in actuality, attempting aspiration and irrigation delays definitive treatment. Our preferred initial approach after 48 hours duration is a T-shunt or T-shunt with tunneling.
Surgical techniques
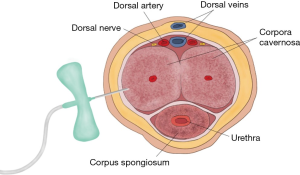
Shunting procedures, described as distal (Figure 4), proximal (Figure 5) or venous (Figure 6), channel ischemic blood from the corpus cavernosa to the corpus spongiosum or alternative venous channels. Distal shunts allow blood flow from the corpus cavernosa to the glans penis and include the Winter’s (biopsy needle), Ebbehoj (#11 blade knife), T-shunt (#10 blade knife) and Al-Ghorab (excision of the distal tip of the corpus cavernosum through incision into the glans). Proximal shunts allow blood flow from the proximal corpora to the spongiosum and include the Quackels (perineal) and the Sacher (penoscrotal) techniques. Venous shunts allow blood flow from the corpora to a vein and include the Greyhack (saphenous vein) and the Barry (dorsal vein of the penis). The AUA guidelines (1) include descriptions of the Winter (7), Ebbehoj (8), Al-Ghorab, Quackels (9), and Grahyack (10) shunts. The EAU guidelines (2) include these shunting procedures in addition to the T-shunt (11) and the Burnett tunneling technique (6).
T-shunt and corporal tunneling
Transgranular T-shunt (Figure 7) is the sole surgical shunting procedure in our treatment algorithm as it is most efficient and effective method, and it has the fewest associated surgical risks. The technique is easily learned as the anatomy is familiar to all urologists and emergency room physicians. The procedure can be performed under local anesthesia in a clinic or ER or in the operating room with sedation plus local anesthesia or general anesthesia, depending on patient and surgeon preference. It is important to obtain written informed consent prior to performance of any procedure for priapism with emphasis placed on the risks of erectile dysfunction (ED), change in penile sensation, glanular hypoesthesia, poor cosmesis of the surgical incision, and the risk of need for repeated procedures.
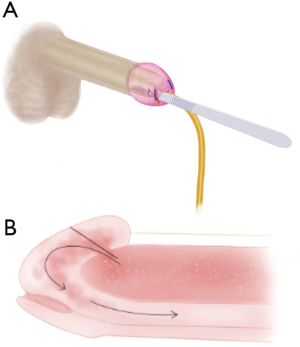
Description of T-shunt procedure and tunneling
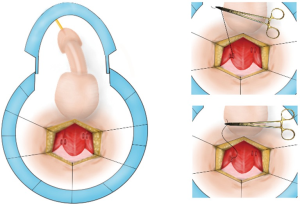
When a shunt is deemed necessary, a unilateral-T shunt is performed initially in most cases. To perform the procedure, the rigid distal tips of the corpora are palpated through the flaccid glans. Lidocaine 1% or bupivacaine 0.25% is injected subcutaneously into the glans penis and circumferentially at the base of the penis. A 10-blade scalpel is positioned vertically parallel to the meatus of the penis, inserted through the glans into the distal tip of the corpus cavernosum, and advanced into the corpus until the hub of the blade is at skin level. The sharp edge of the blade is then rotated 90-degree laterally and withdrawn through the glans (12). Upon initial incision, there is typically an immediate return of copious dark blood. The ischemic blood is expressed manually until the return of bright red blood, which suggests that the flow of oxygenated blood has been reestablished. This procedure should lead to immediate detumescence such that the contralateral distal corporal tip is no longer palpable. This occurs because the corpora are in continuity, joined via the incomplete midline septum. Next, the glans wound is closed with running locking absorbable suture, such as 4-0 or 5-0 chromic gut. Sutures should be placed superficially to avoid occluding the shunt with deeply placed sutures. Interrupted sutures are not ideal as the glans has a rich blood supply and bleeding may persist. The patient is observed for 15 minutes. If priapism recurs, the T-shunt procedure should be performed on the contralateral side.
On the other hand, if the dark ischemic blood drains out sluggishly and the penis remains partially erect after expression of ischemic blood (due to severe edema of the intracavernous tissue), bilateral transgranular intracorporal tunneling should be performed through the glans incisions (Figure 8). A 20–22 French (approximately 7 mm) straight urethral dilator (female sounds) should be advanced slowly, parallel to the long axis of the penis, and angled slightly laterally to minimize the risk of urethral injury. The sound should be advanced through the cavernous tissue to the base of the penile shaft. This procedure creates a large tunnel allowing more effective evacuation of old blood as well as passage of arterial blood to the distal penis to keep the shunt open (13). Next, ischemic blood is manually expressed from the incision site(s) until bright-red blood in seen. Finally, the incisions are closed with superficial running locking 4-0 or 5-0 chromic gut.
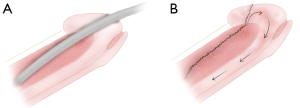
After 15 minutes, the patient should be reassessed to ensure that priapism has resolved. For episodes lasting longer than 48 hours, it is typical to observe post-ischemic hyperemia, a state in which the penile shaft is firm from local edema despite evacuation of trapped ischemic blood from the corpora. If the patient reports resolution of the constant dull ache within the penis and if the penis is sufficiently soft that the shaft indents 1–1.5 cm when compressed, the shunt is considered patent and no further procedures are necessary. When in doubt, a color duplex ultrasound (not intracavernous blood gases) should be performed as described above. The presence of flow in the cavernous artery indicates that the shunt is open and the tumescence is due to post-ischemic hyperemia and tissue edema; no further intervention is needed. If the penis returns to a rigid state with no flow in the cavernous artery, the tunneling procedure should be repeated bilaterally through the same glanular incisions (14). Using this algorithm, we have not had an incidence in which a “traditional” proximal shunting procedure was required as corporal tunneling essentially establishes a proximal shunt.
After completion of the procedure, the glans incisions are closed as described above and coated with antibiotic ointment for 5 days. No compressive dressing should be applied as these dressings tend to become progressively tighter and may lead to compression of the dorsal vein and/or corpus spongiosum, occluding the shunt. These dressings may also cause ischemia if applied too tightly.
Complications during and after shunting procedures
Distal shunt complications
After creation of a corporoglanular (distal) shunt, a larger-than-normal volume of arterial inflow into the corpora cavernosa is expected. This reactive post-ischemic hyperemia, which may last for hours to days, requires a large caliber shunt to move the large volume of inflow. If the shunt is small, the intracavernous pressure will rise and compress the surrounding tissue, obstructing the shunt and leading to recurrent priapism. A small shunt, such as created by an #11 blade scalpel (Ebbehoj shunt) or biopsy needle (Winter shunt), may close spontaneously due to the small caliber. For creation of the T-shunt, it is recommended that a #10 blade knife be utilized and rotated 90 degrees laterally as it is withdrawn to maximize the size of the shunt. Furthermore, when the blood is exposed to the collagen of the incised tunica albuginea, the clotting cascade begins and the shunt may clot if the patient is not on anticoagulation therapy prior to and after shunt creation.
Failure of a T-shunt created using a #10 blade may be caused by severe local tissue edema impeding flow across the shunt. This happens less frequently since we began to treat with antiplatelet agents routinely. In cases of refractory priapism where initial #10 blade T-shunt with tunneling failed, repeated #10-blade T-shunt with tunneling has been successful after the patient is anticoagulated.
An additional risk associated with Al-Ghorab distal shunts is damage to dorsal nerve branches or the dorsal arteries during glanular incision. This can lead to glans numbness or necrosis, respectively.
Proximal shunt complications
Proximal shunts drain blood from the proximal corpus cavernosum to the proximal corpus spongiosum and urethral bulb either through a perineal incision (Quackles) or a penoscrotal (Sacher) incision. These shunts are unnecessarily complex to preform, are time consuming, and carry increased procedural risks. Distal T-shunt with tunneling as described above has replaced the need to perform these shunts entirely. Anatomically, the sponge of the penile urethra is very thin in comparison to the sponge at the level of the bulbar urethra. If a shunt is created through a penoscrotal incision, the thin layer of spongiosal tissue present is inadequate to drain the cavernosum and escalates the risk of urethral injury. Cavernositis, caverno-urethral-cutaneous fistula, complete occlusion of the urethra, and profound cavernosal fibrosis may occur when a proximal shunt is performed too distally. As the distal T-shunt with tunneling is a simple, highly efficacious technique, there is no need for proximal shunting procedures.
Venous shunt complications
Creation of a venous shunt requires expert microsurgical skills. In the case of the saphenous vein shunt (Greyhack), a second incision is required for dissection of the saphenous vein which increases peri-operative and post-operative morbidity and raises the risk of post-operative wound complications. The path of the shunt is also meandering and may lead to poor outflow through the graft. It is also feasible that the patient may need the saphenous vein in the future for coronary artery bypass grafting surgery.
The dorsal vein graft (Barry) is a more direct graft that does not require a second incision. These procedures require degloving of the penis or a penile shaft incision and incision into the tunica albuginea of the shaft which carry the risks of wound healing complications and sensation loss, especially if the patient has had previous penile surgery. In the short term, it is also possible that the patient may occlude his graft with clot, in which case another procedure would be necessary. In the long term, it is possible that the graft might remain patient in which case a surgical procedure for ligation of the shunt would be required to prevent erectile dysfunction.
Post-operative management
After any shunting procedure, the patient should continue anticoagulation therapy with both aspirin 81 mg and clopidogrel 75 mg by mouth daily for 5 days. This will ensure that the surgically created shunt remains patent allowing for continued circulation of blood from the corpus cavernosa to the corpus spongiosum. After 5 days, the post-ischemic hyperemia typically has resolved, and it is prudent to cease treatment with antiplatelet agents so that the shunt will close promptly. If the shunt remains patent beyond the desired time frame, it is possible that the shunt will mature into a fistula that causes permanent ED. This situation may necessitate surgical intervention to close the fistula and restore erectile function.
Post-shunt hyperemia
In many cases of ischemic priapism that last longer than 24 hours, post-shunt semi-tumescence persists even after a successful shunting procedure. This is the result of reactive hyperemia in response to the extended hypoxic and acidotic state. This condition is generally painless and may continue if the shunt remains open. Once the hyperemic state subsides, the normal minimal-flow state of the flaccid penis will not be able to keep the shunt open, leading to spontaneous closure in most cases.
ED
In the AUA guidelines, based on expert consensus, the rate of ED is quoted as <25% for distal shunts and 50% for proximal shunts (1). In our experience, the T-shunt plus perioperative anticoagulation has been very successful in reversing ischemic priapism and preserving erectile function. If the episode of priapism lasted less than 48 hours, permanent ED as a consequence is unlikely. Even if the episode lasted longer than 48 hours, if interventions were performed during that time period to temporarily restore penile circulation (repeated aspiration/irrigation, shunt, etc.), the “longest duration without intervention” should be used to estimate the possibility of ED for patient counseling purposes. We have several patients who presented with 5 to 11 days duration of priapism but underwent repeated aspiration and injection of adrenergic agent by local urologists. Erectile function was fully recovered 2–3 months after successful T-shunt plus perioperative anticoagulation as the true ischemic period was interrupted by periods of restored penile flow.
Early penile prosthesis implantation
Early malleable prosthesis implantation with the option for later placement of an inflatable prosthesis has been proposed (15,16). The hypothetical advantage of this approach is the avoidance of scarred, fibrotic corpora at the time of implantation, which can lead to a higher risk of surgical complications, such as proximal perforation or urethral injury due to difficulty with distal dilation, in addition to loss of penile length and girth. In our clinical experience with T-shunt + perioperative anticoagulation, it is not uncommon for younger patients to recover varying degrees of erectile function even if the duration of ischemic priapism was longer than 48 hours. These patients may need oral PDE5 inhibitors or intracavernosal injection with vasoactive agents to augment function. Severe or complete ED after a priapism episode will be apparent within weeks of the episode, and the decision for either inflatable or malleable penile prosthesis placement can be made at that time without significant penile fibrosis or loss of penile length and girth.
In cases of refractory priapism where primary attempt at shunting has failed, it is possible that placement of a malleable prosthesis might lead to distal erosion of the device depending on the size of the tunica defect. This would be a disaster for both the patient and the urologist.
Summary
Following a duration- and physiology-based algorithm simplifies treatment of this perplexing condition and maximizes outcomes for patients. We hope that the current discussion of our treatment algorithm and the missteps that we have experienced will lend urologists confidence in treating ischemic priapism.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol 2003;170:1318-24. [Crossref] [PubMed]
- Salonia A, Eardley I, Giuliano F, et al. European Association of Urology guidelines on priapism. Eur Urol 2014;65:480-9. [Crossref] [PubMed]
- Kovac JR, Mak SK, Garcia MM, et al. A pathophysiology-based approach to the management of early priapism. Asian J Androl 2013;15:20-6. [Crossref] [PubMed]
- Lue TF, Garcia M. Should perioperative anticoagulation be an integral part of the priapism shunting procedure? Transl Androl Urol 2013;2:316-20. [PubMed]
- Brant WO, Garcia MM, Bella AJ, et al. T-shaped shunt and intracavernous tunneling for prolonged ischemic priapism. J Urol 2009;181:1699-705. [Crossref] [PubMed]
- Burnett AL, Pierorazio PM. Corporal "snake" maneuver: corporoglanular shunt surgical modification for ischemic priapism. J Sex Med 2009;6:1171-6. [Crossref] [PubMed]
- Winter CC. Cure of idiopathic priapism: new procedure for creating fistula between glans penis and corpora cavernosa. Urology 1976;8:389-91. [Crossref] [PubMed]
- Ebbehoj J. A new operation for priapism. Scand J Plast Reconstr Surg 1974;8:241-2. [Crossref] [PubMed]
- Quackels R. Treatment of a Case of Priapism by Cavernospongious Anastomosis. Acta Urol Belg 1964;32:5-13. [PubMed]
- Grayhack JT, McCullough W, O'Conor VJ Jr, et al. Venous Bypass to Control Priapism. Invest Urol 1964;1:509-13. [PubMed]
- Lue TF, Pescatori ES. Distal cavernosum-glans shunts for ischemic priapism. J Sex Med 2006;3:749-52. [Crossref] [PubMed]
- Garcia MM, Shindel AW, Lue TF. T-shunt with or without tunnelling for prolonged ischaemic priapism. BJU Int 2008;102:1754-64. [Crossref] [PubMed]
- Segal RL, Readal N, Pierorazio PM, et al. Corporal Burnett "Snake" surgical maneuver for the treatment of ischemic priapism: long-term followup. J Urol 2013;189:1025-9. [Crossref] [PubMed]
- Garcia M, Porten S, Lue TF. Commentary on refractory ischemic priapism. Transl Androl Urol 2012;1:61-5. [PubMed]
- Zacharakis E, De Luca F, Raheem AA, et al. Early insertion of a malleable penile prosthesis in ischaemic priapism allows later upsizing of the cylinders. Scand J Urol 2015.1-4. [PubMed]
- Zacharakis E, Garaffa G, Raheem AA, et al. Penile prosthesis insertion in patients with refractory ischaemic priapism: early vs delayed implantation. BJU Int 2014;114:576-81. [Crossref] [PubMed]