Correlation of self-reported urologic symptoms with systemic health conditions in minority men
Introduction
While men are less likely to seek preventative primary care or care for new symptoms (1), the onset of urologic symptoms such as benign prostatitis hyperplasia (BPH)/lower urinary tract symptoms (LUTS), erectile dysfunction (ED) and chronic pelvic pain syndrome (CPPS) will often prompt urologic consultation. These urologic symptoms may often be caused or exacerbated by disorders in non-urologic systems such as cardiovascular, endocrine, psychologic and neurologic as well as conditions such as obesity and obstructive sleep apnea (OSA). Indeed, the urologic complaint may be the first clinical interaction that unearths cardiovascular disease (2), diabetes (3), OSA (4) or multiple sclerosis (5).
While prior studies have linked prevalence of LUTS, ED and CPPS in men (6-8), as well as association of individual systemic conditions with individual urologic symptoms (9), there has not been an attempt to link multiple conditions together in a true “men’s health phenotype”. Furthermore, in such studies participation of minority men with limited access to healthcare is typically low, and indeed their burden of undiagnosed co-morbid conditions may be high.
The purpose of this study was to obtain self-reported urologic symptoms and systemic illnesses from adult men attending a Men’s Minority Health Fair. Our hypothesis was that a greater burden of systemic illness would be associated with a higher incidence and severity of urologic complaints.
Methods
Questionnaires were distributed at the Cleveland Clinic annual Men’s Minority Health fair in April 2017 under an IRB approved protocol. Urologic symptoms were assessed with the International Prostate Symptom Score (IPSS) (10), Sexual Health Inventory for Men (SHIM) (11) and NIH Chronic Prostatitis Symptom Score (CPSI) (12). Each was graded as absent/mild [0], moderate [1] or severe [2] by standard criteria for each (Table 1) and totaled for a urologic score (US). Other questions included age, height/weight and queried heart disease, diabetes, anxiety/stress, sleep apnea and neurologic disease. A systemic score (SS) graded each plus obesity for 6 domains (0–2 for each) (Table 1).
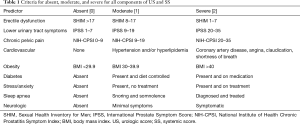
Full table
Since distribution of the US was not normal, Spearman Rho was used to assess correlation between systemic domains and the total SS with the US. For multivariable analysis, ordinal logistic regression was performed using R 3.4.0. Significance was set at P<0.05.
Results
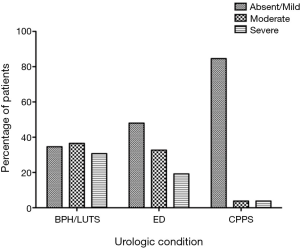
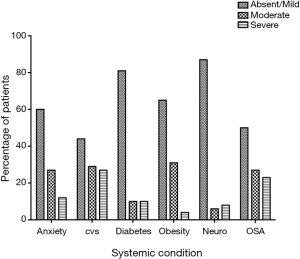
A total of 52 men completed the surveys with a mean age of 58.8±9.6 (range, 37–76) years. By symptom score criteria, 17 (33%) had 1 urologic condition, 19 (37%) had 2 and 5 (10%) had all 3. The presence and severity of urologic symptoms is plotted in Figure 1 with BPH/LUTS being most common, followed by ED and then CPPS. Mean total US was 1.9±1.6 (range, 0–6) and mean SS was 2.9±2.6 (range, 0–10). Presence and severity of systemic symptoms is plotted in Figure 2 with Cardiovascular being the most common. Men with a higher US [3–6] were older than men with a lower US [0–2] (65.5±1.4 vs. 56.6±1.5 years, P=0.003).
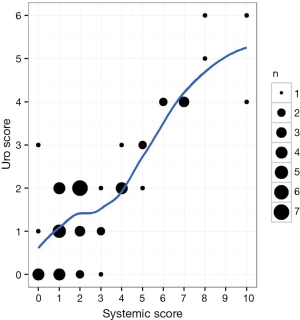
There was a strong positive correlation between US and SS (Spearman Rho =0.73, P<0.0001) (Figure 3). The hierarchy of systemic condition impact on US was cardiovascular > anxiety > obesity > diabetes > sleep apnea > neurologic. By multivariable analysis, after adjusting for age, each systemic component strongly correlated with the US. The multivariable model with age plus all of the systemic scores predicted US more accurately than with any one of its components alone with an odds ratio of 4.01 (2.43–6.63, P<0.0001) and area under the receiver operating characteristic curve of 0.863 (Table 2).
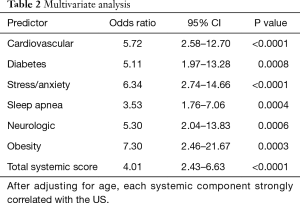
Full table
Discussion
The relationship between urologic men’s health conditions and specific systemic diseases and health conditions is well established. Anxiety and alarm falsification can exacerbate pain and LUTS (13). Cardiovascular disease and its treatments can cause ED and LUTS and possible CPPS (14). Diabetes can cause both ED through vascular disease and association with low testosterone and LUTS through bladder cystopathy and osmotic diuresis (15). Newer diabetes drugs can also exacerbate LUTS. Obesity is associated with LUTS and ED (16) and weight loss can improve both conditions (17). Neurologic conditions can interact in multiple ways; iatrogenic severed nerves lead to ED, inflamed nerves to pelvic pain (18) and interrupted nerve pathways to neurogenic bladder. Finally, OSA is also associated with ED (4), LUTS (19) and CPPS (20).
Given these associations, a man presenting with urologic complaints has a high chance of at least some of the symptoms being related to chronic systemic illnesses, either through confounding symptoms or through direct etiology. In this study of minority men presenting to a free health fair, we found that self-reported systemic conditions correlated very strongly with the presence and severity of urologic symptoms. This was true for each of the six conditions studied in both univariate and multivariable analyses controlled for age and indeed, by logistic regression, the model with all systemic conditions included was more predictive than any of the individual domains. What are the practical corollaries to these findings? First, that men presenting with new severe urologic symptoms, especially those with poor access to preventative health care (21), may be at risk for having undiagnosed systemic conditions. Indeed, a recent meta-analysis concluded that men with moderate to severe LUTS where at increased risk for subsequent cardiac events (22). Second, and more importantly for the Urologist, failure to identify and treat these comorbid conditions along with the urologic complaints has the chance to reduce the effectiveness of medical or surgical interventions for the primary urologic complaint.
The major limitations to this study are the modest numbers of respondents and the self-reported nature of the systemic conditions. It is likely that several conditions such as hypertension, diabetes and sleep apnea were under-diagnosed in this group of medically under-serviced men. We are currently creating a more robust men’s health phenotype based upon clinical data that includes bloodwork (i.e., HbA1c, serum testosterone) which will hopefully capture this information more accurately.
In conclusion, self-reported systemic health conditions correlate strongly with presence and severity of urologic symptoms in minority men.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Questionnaires were distributed at the Cleveland Clinic annual Men’s Minority Health Fair in April 2017 under an IRB approved protocol (IRB 17-401). Written informed consent was obtained for publication of this manuscript and any accompanying images from all men who completed the surveys.
References
- Jeffries M, Grogan S. Oh, I’m just, you know, a little bit weak because I’m going to the doctor’s’: young men’s talk of self-referral to primary healthcare services. Psychol Health 2012;27:898-915. [Crossref] [PubMed]
- Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA 2005;294:2996-3002. [Crossref] [PubMed]
- Nandy PR, Saha S. Association between components of metabolic syndrome and prostatic enlargement: An Indian perspective. Med J Armed Forces India 2016;72:350-5. [Crossref] [PubMed]
- Taken K, Ekin S, Arısoy A, et al. Erectile dysfunction is a marker for obstructive sleep apnea. Aging Male 2016;19:102-5. [Crossref] [PubMed]
- Torelli F, Terragni E, Blanco S, et al. Lower urinary tract symptoms associated with neurological conditions: Observations on a clinical sample of outpatients neurorehabilitation service. Arch Ital Urol Androl 2015;87:154-7. [Crossref] [PubMed]
- Nakamura M, Fujimura T, Nagata M, et al. Association between lower urinary tract symptoms and sexual dysfunction assessed using the core lower urinary tract symptom score and International index of erectile function-5 questionnaires. Aging Male 2012;15:111-4. [Crossref] [PubMed]
- Nickel JC, Elhilali M, Vallancien G. Benign prostatic hyperplasia (BPH) and prostatitis: prevalence of painful ejaculation in men with clinical BPH. BJU Int 2005;95:571-4. [Crossref] [PubMed]
- Tran CN, Shoskes DA. Sexual dysfunction in chronic prostatitis/chronic pelvic pain syndrome. World J Urol 2013;31:741-6. [Crossref] [PubMed]
- Kaplan SA, Lee JY, O’Neill EA, et al. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013;16:169-72. [Crossref] [PubMed]
- Barry MJ, Fowler FJ, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549-57; discussion 1564. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-26. [Crossref] [PubMed]
- Litwin MS, McNaughton-Collins M, Fowler FJ, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol 1999;162:369-75. [Crossref] [PubMed]
- Leue C, Kruimel J, Vrijens D, et al. Functional urological disorders: a sensitized defence response in the bladder-gut-brain axis. Nat Rev Urol 2017;14:153-63. [Crossref] [PubMed]
- Shoskes DA, Prots D, Karns J, et al. Greater endothelial dysfunction and arterial stiffness in men with chronic prostatitis/chronic pelvic pain syndrome-a possible link to cardiovascular disease. J Urol 2011;186:907-10. [Crossref] [PubMed]
- Ozcan L, Besiroglu H, Dursun M, et al. Comparison of the clinical parameters of benign prostate hyperplasia in diabetic and non diabetic patients. Arch Ital Urol Androl 2017;89:26-30. [Crossref] [PubMed]
- Chen Z, Miao L, Gao X, et al. Effect of obesity and hyperglycemia on benign prostatic hyperplasia in elderly patients with newly diagnosed type 2 diabetes. Int J Clin Exp Med 2015;8:11289-94. [PubMed]
- Groutz A, Gordon D, Schachter P, et al. Effects of bariatric surgery on male lower urinary tract symptoms and sexual function. Neurourol Urodyn 2017;36:636-9. [Crossref] [PubMed]
- Nickel JC, Atkinson G, Krieger JN, et al. Preliminary Assessment of Safety and Efficacy in Proof-of-Concept, Randomized Clinical Trial of Tanezumab for Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Urology 2012;80:1105-10. [Crossref] [PubMed]
- Bal K, Ayik S, Issi Y, et al. Sleep analysis of patients with nocturia and benign prostatic obstruction. Urology 2012;80:383-8. [Crossref] [PubMed]
- Chung SD, Hung SH, Lin HC, et al. Obstructive sleep apnea and urological comorbidities in males: a population-based study. Sleep Breath 2016;20:1203-8. [Crossref] [PubMed]
- Tucker-Seeley RD, Mitchell JA, Shires DA, et al. Financial hardship, unmet medical need, and health self-efficacy among African American men. Health Educ Behav 2015;42:285-92. [Crossref] [PubMed]
- Gacci M, Corona G, Sebastianelli A, et al. Male Lower Urinary Tract Symptoms and Cardiovascular Events: A Systematic Review and Meta-analysis. Eur Urol 2016;70:788-96. [Crossref] [PubMed]


