Microdissection testicular sperm extraction
Introduction
Fifteen percent of North American couples present with infertility and male factor infertility plays a role in up to 50% of these couples. About 10–20% of infertile men present with the most severe form of infertility, azoospermia, in which there is a complete lack of sperm in the ejaculate (1). Men with azoospermia must be appropriately evaluated to assess for reversible factors. Following a thorough history, physical exam, and investigations, some patients may be successfully treated medically using hormonal manipulation while others will ultimately require attempts at surgical sperm retrieval. The gold standard for surgical sperm retrieval is microdissection testicular sperm extraction (microTESE).
Etiology and classification of azoospermia
Azoospermia is defined as the absence of spermatozoa in the ejaculate following centrifugation and subsequent microscopy of the specimen on two separate semen analyses. Azoospermia can be classified by etiology into obstructive (OA) and non-obstructive azoospermia (NOA). In OA, while sperm production in the testes is normal, the sperm are unable to reach the ejaculate due to an obstruction occurring somewhere along the male reproductive tract, such as in the epididymis, vas deferens, or ejaculatory tract. On the other hand, in NOA, production of sperm in the testes is impaired, which is typically related to dysfunction along the hypothalamic-pituitary-testicular axis. Central dysfunction may occur due to abnormalities of the hypothalamus or pituitary gland while gonadal dysfunction is due to testicular abnormalities that may be related to a variety of etiologies (Table 1). Ejaculatory dysfunction, such as retrograde ejaculation and failure of emission, can also result in azoospermia.
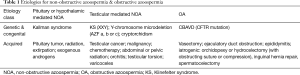
Full table
Evaluation of azoospermia
Evaluation of individuals with evidence of azoospermia on two separate semen analyses should start with a thorough history and physical exam of the male as well as a female fertility evaluation. Male history should assess for the many potential risk factors and etiologies of infertility and azoospermia as outlined in Table 1. Critical domains to cover during history include duration of infertility, coital frequency and technique, prior fertility, childhood and developmental history, history of systemic illnesses, surgical history, sexual history, medication use, and family history of infertility.
A careful physical exam with an emphasis on the genitalia also provides important information on the potential etiology of azoospermia. The general physical exam should assess for degree of virilization, obesity, gynecomastia, anosmia (consistent with Kallman Syndrome), bilateral hemi-anopsia (suggestive of pituitary tumor), and abdominal or inguinal scars. Urogenital exam should include close inspection of the phallus looking for abnormalities of the urethral meatus and penile curvature. Testicular examination should assess for testicular size, consistency, and the presence of masses. Both epididymides and vasa deferens should be palpated for their presence, consistency, and nodularity which may suggest obstructive or infectious etiologies. Finally, palpation of the spermatic cords with and without Valsalva may reveal varicocele while a digital rectal examination may help identify dilated seminal vesicles or cysts causing ejaculatory duct obstruction.
The most important laboratory investigations in the evaluation of the azoospermic male include the semen analysis and serum hormone levels (morning testosterone and FSH at a minimum, but often also LH, prolactin, and estrogen). If the semen analysis demonstrates low pH (<7.2) or absence of fructose, then ejaculatory duct obstruction or congenital vasal absence should be considered and investigated with transrectal ultrasound (TRUS) and physical examination, respectively. Additional investigations for the azoospermic male include genetic testing like karyotype and Y-chromosome microdeletion testing. While none of the investigations can be considered diagnostic of OA versus NOA, the constellation of findings that is highly suggestive of NOA includes small testicular size, normal or elevated FSH levels, abnormal karyotype, and the presence of Y-chromosome microdeletions. Schoor & colleagues determined that an FSH of less than 7.6 mIU/mL and testicular long axis greater than 4.6 cm predicts OA in 96% of cases, and conversely, an FSH of greater than 7.6 mIU/mL with a testicular long axis of less than 4.6 cm predicts NOA in 89% of cases (2). See Figure 1 for a decision flow chart in managing azoospermic patients.
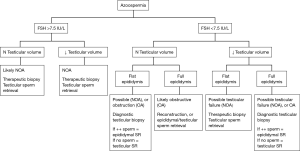
Predictors of sperm retrieval rates (SRRs) in the male patient with NOA
Infertile couples should be counseled regarding the multiple treatment options available for men diagnosed with NOA, which include surgical sperm retrieval for use with IVF-ICSI, the use of donor sperm with IVF, and adoption. For patients wishing to pursue surgical sperm retrieval, it is important to discuss appropriate expectations and reproductive outcomes specific to their particular clinical scenario. Clinical parameters known to affect reproductive outcomes include testicular histology, karyotype, and Y-chromosome microdeletions. In occasional situations, testicular histology may be available and used for counseling when a diagnostic testicular biopsy has been done prior to microTESE, but this information is usually only available after testicular tissue is submitted to histopathology at the time of microTESE. Four testicular histologies are known to cause NOA. Hypospermatogenesis, which is the least severe form of NOA, carries the highest surgical SRR of 73–100% while late maturation arrest has a SRR of 27–86%, early maturation arrest has a SRR of 27–40%, and Sertoli Cell Only syndrome (SCOS), which is the most severe form of infertility, has a SRR of 22.5–41% (3-6).
Men with Klinefelter syndrome (KS) have progressive intratesticular fibrosis and impairment in both testosterone production and spermatogenesis. The mean SRR for men with KS is 66% with clinical pregnancy rates of 49.7% (7). In men with KS, predictive factors for successful sperm retrieval include, LH <17.5 U/L, increase in testosterone to >250 ng/dL following medical therapy, younger post-pubertal age, and normal baseline testosterone: estrogen ratio (8-10). As such, treatment with aromatase inhibitors and selective estrogen receptor modulators may be considered. Men with mixed gonadal dysgenesis (MGD) characterized by the karyotype 45XO, 46XY have limited reports of sperm in the ejaculate or with successful microTESE. Sperm production only occurs in these men that have intact AZFa and AZFb regions in their Y-chromosome. Men presenting with XYY often have azoospermia or severe oligospermia (11) with histology typically demonstrating SCOS or early maturation arrest (12). Men with Y-chromosome microdeletions have differing prognoses depending upon the site of deletion. While no reports of successful sperm retrieval have been described in the literature for those with complete AZFa or AZFb deletions, the isolated USP9Y partial AZFa deletion can result in a variable phenotype ranging from severe oligospermia to azoospermia with Hypospermatogenesis (13,14). Histologically, complete AZFa deletions demonstrate SCOS while AZFb deletions demonstrate SCOS or early maturation arrest (15). Seventy percent of men with AZFc deletions will have sperm in their ejaculate, but typically at very low concentrations less than 1 million sperm per mL (16). The SRRs for azoospermic men with AZFc deletions range between 50–60% (17).
Some factors that have been reported to predict SRRs at microTESE include preoperative FSH levels and testicular volume. We have found that increased FSH and smaller testicular volume do not provide an adverse prognosis for sperm retrieval, since they are not affected by a limited site of sperm production. Some studies have found little association between SRRs and FSH levels, with FSH levels less than 15 IU/mL having a SRR of 51%, FSH levels between 15–30 IU/mL having a SRR of 60%, FSH levels 31–45 IU/mL having a SRR of 67%, and FSH levels greater than 45 IU/mL having a SRR of 60% (18), other studies have demonstrated lower SRRs among men with higher FSH levels (5,19). Different studies have also found conflicting associations between testicular volume and SRRs (5,19).
Optimization prior to microTESE
Medical management
Most men presenting with a central cause of azoospermia due to hypothalamic-pituitary axis dysfunction will dramatically benefit from medical management with gonadotropin releasing hormone (GnRH) or gonadotropins (i.e., hCG, hMG, recombinant FSH). Common regimens include pulsatile GnRH 25–600 ng/kg subcutaneously (SC), intravenous (IV) or by pump every 120 minutes, hCG 1,000–2,500 IU injected intramuscularly (IM)/SC twice per week, or hMG 75–150 IU injected 3 times per week (20), recombinant FSH 75 IU every 1–2 days (21). Most of these men will not require sperm retrieval if testicular function is optimized with hormonal therapy.
Medical optimization strategies have been investigated for patients with azoospermia due to testicular dysfunction, with the goal of increasing both testosterone and FSH levels to stimulate spermatogenesis. A multi-institutional study (22) evaluated the effectiveness of using a combination of clomiphene citrate (CC), hCG, and/or hMG to increase serum testosterone to 600–800 ng/dL and serum FSH to 1.5 times baseline in 612 men with NOA. In this study, the intervention group received hormonal optimization according to a defined protocol while the control group received no hormonal optimization, resulting in a significantly higher SRR for the intervention group (SRR 57% vs. 34% for intervention group vs. control group). While this study supports the medical hormonal optimization of FSH and endogenous testicular testosterone levels to improve SRR, it is important to note that the control group had a lower SRR than previous reports in the literature. Furthermore, a large retrospective study of 1,054 men, did not find benefit of hormonal therapy in men undergoing mTESE. Here, no difference in SRR was identified among men with baseline testosterone >300 ng/dL, compared to men with baseline testosterone <300 ng/dL and not receiving medical hormonal treatment, or men receiving aromatase inhibitors, selective estrogen receptor modulators, or any combination with hCG (23).
Another class of medication that has been used to optimize hormonal parameters in NOA and cryptozoospermia patients is aromatase inhibitors, such as anastrozole and letrozole. Although most studies were performed using anastrozole, which appears to have fewer side effects, one study comparing letrozole to placebo in cryptozoospermia and NOA patients suggested that letrozole may help enhance spermatogenesis with sperm return to the ejaculate in some NOA patients (24). Candidates for management with aromatase inhibitor include men with low serum testosterone (<300 ng/dL) and low testosterone: estradiol ratios (<10), in whom aromatase therapy has been suggested to enhance intratesticular testosterone levels and improve spermatogenesis (1,3,4).
Varicocele repair (VR)
VR has been demonstrated to result in return of sperm to the ejaculate in 10% of patients, thus obviating the need for surgical sperm retrieval (25). VR is more likely to result in sperm return to the ejaculate in patients with hypospermatogenesis or late maturation arrest on testicular histology when compared to more severe NOA histologies (26). Of course, these patients are the same individuals likely to have sperm detected in the ejaculate on a more detailed or repeat semen analysis. A meta-analysis demonstrated that VR increases the likelihood of successful sperm retrieval at microTESE by an odds ratio of 2.65 (27). Other large studies, apparently excluded from the meta-analysis show no effect of prior VR on SRRs. In one series, no increase in post varicocele mTESE SRR was identified and sperm returned to the ejaculate among 22% of men postoperatively, but only 9.6% have adequate motile sperm for ICSI (25). Both female partner age and number of children desired are important considerations when discussing VR in the management of the azoospermic male since the potential benefits of VR are not realized until at least 3–6 months after the repair.
Surgical management of NOA
Following medical optimization in patients with NOA, surgical sperm retrieval can be performed. Several sperm retrieval techniques for NOA have been described in the literature, including testicular sperm aspiration (TESA), sperm mapping where TESA is performed in a systematic grid-like fashion (also called FNA mapping), conventional TESE and microTESE. Systematic review of sperm retrieval techniques has shown that conventional TESE is 2-fold more effective at sperm retrieval than TESA, and microdissection TESE is 1.5-fold more effective at retrieving sperm than conventional TESE. For the purposes of this review we will focus on microTESE, which is accepted by many as the present gold standard for surgical sperm retrieval.
MicroTESE was first described in 1999 and represents an evolution from conventional TESE (28). With the magnification provided by an operating microscope, Dr. Peter Schlegel was able to observe heterogeneity amongst seminiferous tubules within the testicle and noticed that focal areas of dilated seminiferous tubules were more likely to contain areas of active spermatogenesis. By identifying and selectively removing only dilated seminiferous tubules, SRRs increased from 16.7–45% to 42.9–63% (6), with greater numbers of sperm retrieved (160,000 vs. 64,000), and 70-fold less tissue excised (9.4 vs. 720 mg).
MicroTESE technique
MicroTESE is ideally performed under a general anesthetic (Figure 2) . An incision is made through the median raphe to allow easy access to both testes. The larger of two testicles is delivered first by incising through the dartos and tunica vaginalis. Under the operating microscope, the tunica albuginea of the testicle is then incised transversely using a 15-degree micro-knife and taking care to avoid equatorial testicular vessels. Mosquito clamps are placed on each respective side of the tunical incision including the edge of seminiferous tubules to prevent avulsion of the tissue as the testicle is bivalved under gentle pressure from the surgeon’s fingers. Right-handed surgeons should stand to the patients left so that the surgeon’s left hand can be in a more comfortable position between the patient’s legs rather than on the abdomen. A three finger technique is utilized with the left hand both to stabilize the testicle and to maintain exposure. Here, the third digit supports the posterior side of the testis, while the thumb and index finger provide exposure on the cut surface of the testis. The seminiferous tubules are then systematically examined for dilated, opaque tubules. Excellent visualization of the seminiferous tubules is critical and is achieved both by maintaining the seminiferous tubules within the microscope’s focal distance and by ensuring adequate hemostasis using bipolar electrocautery. Once dilated, opaque tubules are identified, the entire length of the centrifugally-oriented tubule is removed with tissue micro-forceps and placed in a small petri dish with sperm transport buffer. After the entire cranial or caudal half of the testis is searched, the tissue is prepared for handoff to an embryologist in the surgical theater who will examine the removed testicular tissue for sperm. If sperm is identified, dissection of the contralateral testicle is unnecessary, but if sperm is not identified, then dissection of the contralateral side proceeds.
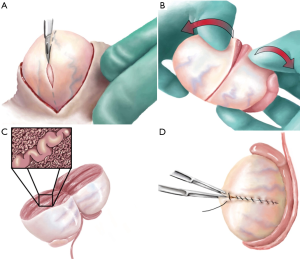
Tissue processing of the excised tubules is essential to optimize successful identification of sperm that are present within the tissue. Excised tubules are minced with scissors until the suspension is fine enough to be aspirated in and out of a 24-gauge angio-catheter prior to inspection by the embryologist. This technique has resulted in a 300-fold increase in sperm recovery (29).
Throughout dissection, hemostasis is carefully maintained using bipolar electrocautery. Once a testis has been fully dissected, series of mosquito clamps are then used to re-approximate the tunica albuginea edges that is then closed using a 5-0 non-absorbable monofilament suture in a running fashion. This serves to mark the location of dissection if a repeat procedure is required in the future. The testicle is returned to its anatomical position within the tunica vaginalis that is then closed with an absorbable monofilament suture in a running fashion. If the contralateral testicle requires dissection, it is performed at this stage in the same manner. Otherwise, the tunica vaginalis is infused with local anesthetic and the dartos layer is closed with an absorbable suture in a running fashion, with care being taken to include the entire cut edge in the closure for optimal hemostasis. Prior to completing the knot, 5 mL of local anesthetic is infused into each hemi-scrotum. The knots are buried within the dartos layer. Finally, the skin is closed with interrupted horizontal mattress stitches and an appropriate dressing is applied.
Complications & considerations
It is important to obtain a semen analysis prior to the planned microTESE, to assess for sperm. Since 5–10% of men with NOA will have sperm in the ejaculate viable for use in ICSI, thus obviating the need for a surgical sperm retrieval. Another important consideration prior to microTESE is the use of fresh or frozen sperm for IVF-ICSI. Among men with NOA that have sperm retrieved, frozen and later thawed, only 33% of sperm will be viable for use with ICSI. Thus, we recommend performing simultaneous ICSI with fresh testicular sperm harvested by microTESE in NOA patients (30). Clinical pregnancy rates with IVF-ICSI using sperm retrieved from microTESE range between 20–50% (31).
Studies have found serum testosterone levels following microTESE to decrease from 316 to 251 ng/dL, but return to 95% of baseline at 18 months, of these 5–10% of men will have a decrease in testosterone significant enough to warrant subsequent androgen replacement. Ultrasound changes in the testes are seen in 18.3% of microTESE testes at 1 month, 10–44% of testes at 3 months, but in only 3.3–10% of testes at 6 months (32,33). Early testicular ultrasound findings following microTESE include hypoechoic changes while late findings at 6 months tend to be limited to focal echogenic lesions of fibrosis and calcification (33). Since time is required for recovery of the limited sperm production that is present in men with NOA, at least 6–12 months should be allowed after microTESE, before considering repeat microTESE procedures if additional attempts are required (34,35). When compared to conventional TESE, microTESE results in lower complication rates, with fewer hematomas, less testicular fibrosis, and less frequent testicular atrophy with higher SRRs (6). We have not experienced testicular loss following microTESE, although some anecdotal reports exist among very small testes.
Acknowledgements
The study was supported by The Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust; American Urology Association New York Section E. Darracott Vaughan MD, Research Scholar Award.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wosnitzer M, Goldstein M, Hardy MP. Review of Azoospermia. Spermatogenesis 2014;4:e28218. [Crossref] [PubMed]
- Schoor RA, Elhanbly S, Niederberger CS, et al. The role of testicular biopsy in the modern management of male infertility. J Urol 2002;167:197-200. [Crossref] [PubMed]
- Bernie AM, Shah K, Halpern JA, et al. Outcomes of microdissection testicular sperm extraction in men with nonobstructive azoospermia due to maturation arrest. Fertil Steril 2015;104:569-73.e1. [Crossref] [PubMed]
- Caroppo E, Colpi EM, Gazzano G, et al. Testicular histology may predict the successful sperm retrieval in patients with non-obstructive azoospermia undergoing conventional TESE: a diagnostic accuracy study. J Assist Reprod Genet 2017;34:149-54. [Crossref] [PubMed]
- Colpi GM, Colpi EM, Piediferro G, et al. Microsurgical TESE versus conventional TESE for ICSI in non-obstructive azoospermia: a randomized controlled study. Reprod Biomed Online 2009;18:315-9. [Crossref] [PubMed]
- Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: a systematic review. Andrology 2014;2:20-4. [Crossref] [PubMed]
- Plotton I, Brosse A, Cuzin B, et al. Klinefelter syndrome and TESE-ICSI. Ann Endocrinol (Paris) 2014;75:118-25. [Crossref] [PubMed]
- Mehta A, Paduch DA. Klinefelter syndrome: an argument for early aggressive hormonal and fertility management. Fertil Steril 2012;98:274-83. [Crossref] [PubMed]
- Rohayem J, Fricke R, Czeloth K, et al. Age and markers of Leydig cell function, but not of Sertoli cell function predict the success of sperm retrieval in adolescents and adults with Klinefelter's syndrome. Andrology 2015;3:868-75. [Crossref] [PubMed]
- Ramasamy R, Ricci JA, Palermo GD, et al. Successful fertility treatment for Klinefelter's syndrome. J Urol 2009;182:1108-13. [Crossref] [PubMed]
- Abdel-Razic MM, Abdel-Hamid IA, ElSobky ES. Nonmosaic 47,XYY syndrome presenting with male infertility: case series. Andrologia 2012;44:200-4. [Crossref] [PubMed]
- Skakkebaek NE, Hultén M, Jacobsen P, et al. Quantification of human seminiferous epithelium. II. Histological studies in eight 47,XYY men. J Reprod Fertil 1973;32:391-401. [Crossref] [PubMed]
- Brown GM, Furlong RA, Sargent CA, et al. Characterisation of the coding sequence and fine mapping of the human DFFRY gene and comparative expression analysis and mapping to the Sxrb interval of the mouse Y chromosome of the Dffry gene. Hum Mol Genet 1998;7:97-107. [Crossref] [PubMed]
- Sun C, Skaletsky H, Birren B, et al. An azoospermic man with a de novo point mutation in the Y-chromosomal gene USP9Y. Nat Genet 1999;23:429-32. [Crossref] [PubMed]
- Krausz C, Forti G, McElreavey K. The Y chromosome and male fertility and infertility. Int J Androl 2003;26:70-5. [Crossref] [PubMed]
- Georgiou I, Syrrou M, Pardalidis N, et al. Genetic and epigenetic risks of intracytoplasmic sperm injection method. Asian J Androl 2006;8:643-73. [Crossref] [PubMed]
- Stahl PJ, Masson P, Mielnik A, et al. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril 2010;94:1753-6. [Crossref] [PubMed]
- Ramasamy R, Lin K, Gosden LV, et al. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril 2009;92:590-3. [Crossref] [PubMed]
- Ghalayini IF, Al-Ghazo MA, Hani OB, et al. Clinical comparison of conventional testicular sperm extraction and microdissection techniques for non-obstructive azoospermia. J Clin Med Res 2011;3:124-31. [PubMed]
- Silveira LF, MacColl GS, Bouloux PM. Hypogonadotropic hypogonadism. Semin Reprod Med 2002;20:327-38. [Crossref] [PubMed]
- Foresta C, Selice R, Ferlin A, et al. Hormonal treatment of male infertility: FSH. Reprod Biomed Online 2007;15:666-72. [Crossref] [PubMed]
- Hussein A, Ozgok Y, Ross L, et al. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int 2013;111:E110-4. [Crossref] [PubMed]
- Reifsnyder JE, Ramasamy R, Husseini J, et al. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2012;188:532-6. [Crossref] [PubMed]
- Cavallini G, Biagiotti G, Bolzon E. Multivariate analysis to predict letrozole efficacy in improving sperm count of non-obstructive azoospermic and cryptozoospermic patients: a pilot study. Asian J Androl 2013;15:806-11. [Crossref] [PubMed]
- Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril 2004;81:1585-8. [Crossref] [PubMed]
- Aboutaleb HA, Elsherif EA, Omar MK, et al. Testicular Biopsy Histopathology as an Indicator of Successful Restoration of Spermatogenesis after Varicocelectomy in Non-obstructive Azoospermia. World J Mens Health 2014;32:43-9. [Crossref] [PubMed]
- Esteves SC, Miyaoka R, Roque M, et al. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl 2016;18:246-53. [Crossref] [PubMed]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999;14:131-5. [Crossref] [PubMed]
- Ostad M, Liotta D, Ye Z, et al. Testicular sperm extraction for nonobstructive azoospermia: results of a multibiopsy approach with optimized tissue dispersion. Urology 1998;52:692-6. [Crossref] [PubMed]
- Schlegel PN, Liotta D, Hariprashad J, et al. Fresh testicular sperm from men with nonobstructive azoospermia works best for ICSI. Urology 2004;64:1069-71. [Crossref] [PubMed]
- Guler I, Erdem M, Erdem A, et al. Impact of testicular histopathology as a predictor of sperm retrieval and pregnancy outcome in patients with nonobstructive azoospermia: correlation with clinical and hormonal factors. Andrologia 2016;48:765-73. [Crossref] [PubMed]
- Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology 2005;65:1190-4. [Crossref] [PubMed]
- Amer M, Ateyah A, Hany R, et al. Prospective comparative study between microsurgical and conventional testicular sperm extraction in non-obstructive azoospermia: follow-up by serial ultrasound examinations. Hum Reprod 2000;15:653-6. [Crossref] [PubMed]
- Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod 1997;12:1688-92. [Crossref] [PubMed]
- Ramasamy R, Schlegel PN. Microdissection testicular sperm extraction: effect of prior biopsy on success of sperm retrieval. J Urol 2007;177:1447-9. [Crossref] [PubMed]


