Scrotal reconstruction and testicular prosthetics
Introduction
Urologists encounter a wide-variety of conditions affecting the scrotum that require surgical management. These range from simple hydrocelectomy to complex scrotal reconstruction. In this review, we briefly discuss hydrocelectomy, which is amongst the most commonly performed procedures by the general urologist. However, it is our hope that we can provide our indications, surgical techniques, and our complications for more complex scrotal procedures, such as scrotoplasty and split-thickness skin grafting, to provide confidence for the urologist who may not routinely perform these surgeries.
Hydrocelectomy
Hydroceles are a commonly encountered urologic condition with an incidence of nearly 1% of adult men (1). Hydroceles have been classified into communicating or non-communicating types—dependent on whether the processus vaginalis remains patent. In adults, hydroceles tend to be non-communicating, and have a variety of causes: idiopathic and reactive (infection, malignancy, trauma). The diagnosis is most commonly made by physical examination and ultrasonography. On physical exam, hydroceles are manifested by a variable, fluid-filled sac which contains the testis. Classically, the scrotal transillumination test has been used to describe the presence of hydrocele or a solid testicular mass. Ultrasonography can be used to confirm the diagnosis (hydrocele, spermatocele), its volume, and its complexity. Management of hydrocele may involve watchful waiting, sclerotherapy, or hydrocelectomy.
Sclerotherapy of hydroceles, commonly performed with concomitant aspiration, involves the injection of a sclerosing agent, such as phenol or tetracycline, into the hydrocele sac. The cure rates of sclerotherapy are variable, with most studies reporting anywhere between 50–95% cure (2-4). A Cochrane review of aspiration and sclerotherapy versus hydrocelectomy revealed surgical management resulted in fewer long-term recurrences, despite higher rates of complications (5). At our institution, sclerotherapy is a seldom-used modality, but remains an option for patients with numerus comorbid conditions that may preclude surgical management.
Hydrocelectomy is one of the most ubiquitous surgical procedures in urologic practice. Although there are numerous techniques for repairing hydrocele (6-8), the two most common procedures are the Jaboulay hydrocelectomy and the Lord procedure (9). At our institution, we prefer the Jaboulay procedure. A small median raphe incision is made and dissected to the level of the hydrocele sac. Once encountered, we use blunt dissection around the tunica to free it from overlying fascia before we deliver it from the incision. It is imperative that the sac is not violated prior, as it makes dissection exceedingly difficult. After the hydrocele sac has been delivered, it is opened with electrocautery, and the fluid is drained in its entirety. We excise as much of the hydrocele sac as possible and evert the remainder without strangulating the cord. The tunical remnant is closed in a running fashion with absorbable suture, and a two-layer closure of the Dartos fascia and scrotal skin is performed. Our dressing consists of triple antibiotic ointment, scrotal fluffs, and tight-fitting underwear for one week following surgery.
Although hydrocelectomy is a straightforward surgery in most instances, there are several important complications worth discussing—namely hematoma and infection. In a series of 110 scrotal procedures, 55% of which were hydrocelectomy, Swartz and colleagues found a 20% complication rate (22/110), with 5% (5/110) hematoma, and surgical site infections in 3.5% (4/110) (10). The most common complication in our practice is scrotal hematoma, which can usually be managed with conservative therapy, such as cold compresses and scrotal support. However, we stress early reoperation for scrotal hematomas. Although a difficult decision to make after a purely elective surgery, it can provide quick and definitive therapy with immediate pain relief. One way we have found to obviate the need for early reoperation is to place a closed-suction drain in the scrotum, especially for hydroceles larger than 300 milliliters. We leave closed-suction drains in place for 5–7 days and place the patient on antibiotics for the length of drain placement. Ordinarily, our antibiotic of choice based on our antibiogram is to utilize sulfamethoxazole/trimethoprim.
Scrotoplasty for penoscrotal web (PSW)
PSW is an inconspicuous medical condition with no consensus as to an exact definition. However, principally, it is a distal attachment of the scrotum to the ventral penile shaft which reduces the penoscrotal angle. Webbing can be congenital but is most commonly acquired following an over-zealous circumcision. Currently, much of the literature regarding PSW in focused in the pediatric population, where the reported prevalence is 4% (11). While PSW is not a pathologic condition, it may affect a man’s perception of penile length, alter penetration during intercourse, and can have profound effects on sexual self-esteem (12). El-Koutby and Amin suggested a tiered classification system of grading PSW: Grade #1 web extends to proximal third of penile shaft; Grade #2 extends to the mid third; and Grade #3 extends to the distal-most third (11).
More recently, attention has been turned to adult PSW, specifically in the context of erectile dysfunction and penile prosthetic surgery. Although patients undergoing penile implantation are counseled that stretched penile length is the best predictor of post-implantation erect length, penile shortening following device insertion remains a common complaint (13,14). Thus, a number of strategies have been reported in regards to increasing perceived penile length at the time of penile implantation and many of these reconstructive techniques focus on scrotoplasty/ventral phalloplasty performance (15,16).
Perhaps the simplest scrotoplasty technique involves making a horizontal scrotal incision with closure in the longitudinal axis (17). Additionally, ventral phalloplasty can be completed at the time of penile prosthetic implantation with good results, a practice that we employ frequently at our institution (18). For more complicated and severe PSW deformities during penile implantation, Carrion and associates described utilizing opposing “checkmarks” to excise redundant scrotal tissue near the penoscrotal junction, with proximal margins one centimeter away from the penile shaft (19). The curved nature of the incision helps decrease the amount of unopposed tissue that cannot be closed. This technique has been demonstrated to have high patient satisfaction, and the majority (84%, 36/43) of patients in this large series reported increased perception of penile length (18).
A single or double Z-plasty can also be utilized as both increase the longitudinal length of skin and give the appearance of increased penile length. The main limb of the Z-plasty is placed on the median raphe, and all limbs should be of equal length and at the same acute angles (20). However, a common criticism of the Z-plasty is that the apices carry too much tension and might have poor blood supply thus making the wound more prone to breakdown. The V-Y advancement scrotoplasty is another recently described contemporary maneuver to potentially avoid some of the limitations of the Z-plasty by maintaining the underlying blood supply. In this approach, a V-shaped incision with its apex at the penoscrotal junction is created, mobilized, and then closed such that a Y-configuration is formed by the suture line (21). Each time an advancement is formed, the length gained is approximately 0.4 times that of the vertical limb (21). At our institution, we perform a variation of the previous technique called a V-Y flap scrotoplasty (VYFS) in which the apex of an inverted V-shaped incision is oriented toward the urethral meatus and mobilized. As the inverted V is mobilized caudally, it can then be tailored, amputated, and closed in a longitudinal axis based on the severity of PSW (Figure 1). We stress a three-layer wound closure with great attention paid towards reducing any tension on the skin closure layer. In summary, we utilize two running layers of Dartos with a running 3-0 Monocryl (Ethicon, Inc., Piscataway, NJ, USA) suture, and skin with interrupted 3-0 Chromic catgut (Ethicon, Inc., Piscataway, NJ, USA) suture. We have found that performance of an interrupted skin closure rather than a running closure leads to less overall wound breakdowns as any tension on the wound is distributed over a number of individual sutures instead of on a continuous running suture line.

Overall, performance of scrotoplasty is an important tool in the armamentarium of general and reconstructive urologists that gives patients a high level of satisfaction. For concomitant scrotoplasty and penile implant cases, we most certainly caution surgeons to avoid an over-exuberant scrotoplasty incision as this might lead to a closure on tension and subsequent wound breakdown. Such wound breakdowns risk device exposure and a subsequent need for device removal.
Incidence and etiology of scrotal skin loss
Scrotal skin loss is an uncommonly encountered condition, and its etiology is wide-ranging: scrotal/perineal infections [Fournier’s gangrene (FG)], trauma (blunt, penetrating, burn), lymphedema (congenital, acquired, infectious), hidradenitis suppurativa (HS), and genital cancers, amongst others. After initial management of all such conditions, a thorough understanding of complex reconstructive maneuvers is mandatory prior to undertaking a cosmetic repair.
FG is an often-insipid, gangrenous infection of genital/perineal soft tissue. Its estimated incidence is reported to be 1.6 per 100,000 males (22), with an almost 10:1 predilection over females (23). The local infection causes microbial toxin release which directly breaks-down soft tissues, as well as forcing small arterial and venous thrombi which lead to further tissue hypoxia and breakdown (24,25). A number of host factors are known to be associated with FG, and likely contribute to an immunocompromised state, making infection more severe, including: diabetes mellitus, HIV, blood dyscrasias, alcoholism, chronic steroid use, iatrogenic immunosuppression (e.g., transplant patients), or malignancy requiring chemotherapy (24-26). Diagnosis of FG is often multimodal and includes history, physical exam, routine labs, and/or scrotal ultrasound, and computed tomography (CT). The pathognomonic or classical physical exam finding is crepitus, which indicates the presence of subcutaneous gas, and is present in up 64% of patients (27).
Although genitourinary trauma comprises up to 10% of all patients presenting with other traumatic issues, scrotal/testicular trauma comprises 27.8% to 68.1% of those cases (28,29). Scrotal trauma encompasses both blunt and penetrating injuries, animal/human bites, and burns. The majority of scrotal trauma is blunt in nature (85%) (30), while gun-shot wounds (GSWs) (55%), stab wounds (42%), and bites (3%) account for the remaining 15% of injuries (30). Genital burn injuries are relatively rare, as the penis and scrotum are mobile, and protected on either side by the lower extremities. In patients presenting to burn centers, the reported incidence was 2.8% to 13%, and the most frequent causes include: flames (24–77%), hot liquids (15–64%), and chemicals (8–16%) (31,32).
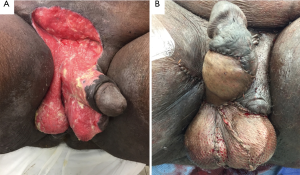
Genital lymphedema encompasses a wide-variety of pathologies, and can present as mild edema or overt elephantiasis (Figure 2). Typically, it is categorized based on its time of onset, underlying pathology, or by its location (penile, scrotal, penoscrotal). As it is an amalgamation of multiple underlying disease processes, its true incidence is difficult to discern. Underlying causes include: neoplasm, infection, radiation therapy, iatrogenic, and congenital, amongst others (33,34). Neoplasm, such as prostate, bladder, penile, colorectal, gynecologic, or hematologic can cause malignant infiltration of inguinal lymphatic channels with resultant dilation of distal vessels. Prostatectomy with pelvic lymph node dissection may carry an up to 15–30% risk of lower extremity lymphedema, and 50% of these patients may have involvement of penis/scrotum (35). Patients with scrotal lymphedema may be present with slowly- or rapidly-progressing edema of the scrotum, depending on pathology. Diagnosis is usually made with history and physical examination, although occasionally imaging using ultrasound, CT, or magnetic resonance imaging (MRI), or lymphoscintigraphy may be needed to diagnose underlying cause.

HS is a debilitating, chronic inflammatory disease of apocrine glands, commonly in the axilla, groin/perineum, and gluteal clefts. Although not frequently discussed, its incidence is anywhere between 1–4% of the population (36), more frequently affecting those in their third decade. Perineal and genital involvement occurs in 24% of all patients with HS, and may recur in up to 75% (37). Important risk factors implicated in the development of HS include tobacco smoking, obesity and a positive family history (38). HS typically presents with an insidious nature, with small areas of pain, erythema, or tender nodularity and can progress to large, sometimes coalescent abscesses, which lead to sinus-tract formation, progressive fibrosis, and local lymphedema.
Management of conditions requiring scrotal skin resection
The initial management of scrotal skin loss is largely dependent on the inciting disease process. In order to appropriately manage FG, genital lymphedema, and hidradenitis, surgical principles mandate the resection of affected genital skin with either immediate or eventual reconstruction of the wound defects. FG, in particular, carries with it substantial morbidity, and a reported mortality between 20–40% in historical series, although this is likely an overestimation in contemporary practice (22). As such, the necrotizing soft tissue infection must be treated early and aggressively. Immediate management includes hemodynamic monitoring, fluid resuscitation, and administration of broad-spectrum antibiotics with adequate coverage of gram-positive, gram-negative, aerobic, and anaerobic bacteria. We prefer a common antibiotic regimen consisting of gentamicin, metronidazole or clindamycin, and a third-generation cephalosporin (ceftriaxone, ceftazidime, etc.); unusual causes may require the use of alternative antibiotics or antifungals. FG requires emergent and radical surgical debridement with irrigation of all non-viable tissue until healthy, bleeding edges are encountered. However, it is almost always necessary to perform multiple debridements and we often recommend at least one “second look” visit to the operating room to thoroughly examine the patient and assess the viability of the tissues with the patient asleep (39). At our institution, our second-look procedures serve to validate our previous debridements; if we encounter additional non-viable tissue, we proceed to additional take-backs until no additional debridement is necessary. In highly aggressive cases, a multidisciplinary approach with general surgery colleagues may be required in the event of abdominal involvement as loop colostomy performance might be necessary. However, at our institution, we manage all genital wounds primarily; for extensive wounds that start in the genitalia and proceed into the abdomen, we have involved plastic surgery for management of the abdominal component. Another acceptable management strategy might call for urologists to debride the patient’s genitalia as much as is indicated prior transitioning all wound management to plastic surgery colleagues.
Due to the robust blood supply to the testicles, orchiectomy during FG presentation is only rarely indicated (40,41). In cases of extensive scrotal involvement, the use of anteromedial thigh pouches was historically described in order to house the testicles and preserve viability and future fertility. This is accomplished first by complete mobilization of the testes and spermatic cord to the external inguinal ring for adequate length. The thigh pouches are then created on the anteromedial thigh using blunt dissection to expose the fascia lata. As described historically, the testicles are to be placed within the pouch anteriorly, and at differing levels such that rubbing and local trauma is avoided when the patient is moving their lower extremities. Importantly, though, we feel thigh pouches to be an unnecessary step in FG management as this historic technique is often not necessary in contemporary practice. Further, in our experience, patients with thigh pouches tend to have significant groin pain and discomfort while walking regardless of anterior location of the pouches. Instead, we prefer to keep the testicles wrapped with saline gauze or included in the vacuum-assisted closure (VAC) dressing of the entire FG wound.
Following surgical debridement, local wound care is paramount and consists of several options: wet-to-dry (WTD) dressings with frequent changes (BID or TID) and/or the use of negative-pressure vacuum therapy or VAC. We usually prefer to initially manage all patients with WTD dressings prior to the “second look” operation. Once tissue viability is confirmed at the second operation and no additional debridement is required, we often perform VAC therapy with a plan of changing the VAC every 48–72 hours. Depending on severity of the infection and patient comfort, we have often brought patients to the operating room simply for wound inspection and VAC change under anesthesia—as successful VAC placement is predicated on having a good seal for the VAC sponges. The use of the wound-VAC confers multiple advantages to the patient and surgeon alike: a theoretically faster rate of granulation tissue formation, reduced microbial load, fewer dressing changes, and faster wound healing (42-45). Usual settings for VAC therapy are continuous suction at 125 mmHg, as higher pressures may decrease local blood flow. Importantly, urologists may struggle in maintaining suction to 125 mmHg due to the various creases in and around the genitalia. In these cases, we have had success with placing the wound VAC to wall suction in our inpatient population. Once the serial debridements have been performed and the patient has been stabilized, the urologist is often confronted with a large defect that requires closure (Figure 3). Our preference is to wait at least a period of 7–10 days prior to undertaking skin graft closure of genital wound defects in FG patients.
In contrast to FG, scrotal injuries caused by trauma, burns, and occasionally bites are initially managed as per ATLS algorithms, and are usually found on secondary or tertiary survey. Typically, once the patient is stable hemodynamically and concerns over other visceral injuries have ruled out, attention can be turned to the scrotum. Scrotal wounds should be copiously irrigated with normal saline and all visible debris should be removed. If indicated, the patient should receive antimicrobial and tetanus prophylaxis. Scrotal injuries caused by burns, penetrating trauma, or infection should undergo local exploration and debridement per the AUA urotrauma guidelines (46). If there is any indication of Dartos violation, we have an extremely low-threshold to proceed for scrotal exploration since missed testicular injuries could result in orchalgia, hematoma, or testicular atrophy. However, in cases where the degree of injury is unknown or where we might have an exceedingly low threshold to intervene surgically, we prefer to utilize scrotal ultrasound to rule-out testicular violation. The ultrasound finding of testicular heterogeneity with loss of contour has been shown previously to be highly sensitive and specific for testicular injury (47).
Burns to the genitalia and perineum should be managed similarly to burns elsewhere: removal of substance or clothing that may be contaminated/burning, rapid cooling of tissue to prevent further burns, and local wound care. Non-viable tissue should be debrided until healthy tissue is apparent, however, it appears that conservative management of genital burns may salvage tissue between 61–90% of the time (32). The use of fecal and urinary diversion to promote wound healing, or prevent wound infection in burns or other forms of scrotal skin loss remains controversial and might not be necessary (48). In a review of 1987 patients presenting to the University of Washington Burn Center, Peck and colleagues were able to show that none of their patients required use of indwelling urinary catheter for management of burns, other than acute resuscitation (49). The only instances where we have found urinary and/or fecal diversion to be useful has been in highly refractory and aggressive cases of FG that require extensive and numerous attempts at debridement.
Genital lymphedema is managed conservatively at first, focused on treating the underlying disease process. Initial management includes scrotal elevation and/or compression, and local wound-care once lymphorrhea occurs. In a series of 90 patients presenting with genital lymphedema, Garaffa and colleagues showed that 64% (56/90) were successfully managed with conservative therapy (34). Once local therapy fails, however, the patient may opt for surgical therapy and this consists of complete resection of scrotal epidermis and dermis, which carry the lymphatic channels. Likewise, HS is managed conservatively at first with antibiotic therapy, immunosuppressive agents, and incision and drainage of small abscesses. In a 10-year study of 56 patients undergoing surgical management for their HS, Kagan and colleagues reported roughly two-thirds of patients underwent more than one incision and drainage, and more than 90% were treated with long-term antibiotics (50). Although there are promising trials for targeted immunotherapy as a sole management strategy for HS, surgery remains the mainstay for refractory cases in contemporary practice (51). These findings indicate that conservative measures are likely not curative in nature.
In many non-aggressive and isolated cases of trauma, hidradenitis, and lymphedema, once resection and/or debridement of all involved tissue has been performed, the possibility of primary skin closure needs to be assessed. It is our experience for scrotal pathology in particular that only 40% of the native, uninvolved scrotum needs to be present in order for the urologist to undertake primary closure. In these cases, our recommendation would be to utilize at least 2 to 3 layers of Dartos closure with absorbable suture prior to undertaking skin closure in an interrupted fashion. In most cases of primary scrotal wound closure, it is also our recommendation to leave a closed suction drain for a period of at least 3 days.
Technical considerations of skin grafting for scrotal reconstruction
Once total or near-total (>60%) scrotal skin loss has occurred, there are several reconstructive management options: split-thickness skin grafts (STSG), full-thickness skin grafts (FTSG), or a variety of myocutaneous or fasciocutaneous flaps (52-55). Although there are potential benefits and risks to these approaches, the reality is that outcomes are relatively similar when comparing these approaches (56). There are several important issues to consider prior to scrotal reconstruction, in particular: optimal timing, choice of technique and their effects on sexual function, and after-care. At a minimum, reconstructive efforts should be delayed until all infected or non-viable tissue has been debrided—this effort may take several separate debridements—but in many cases of hidradenitis and lymphedema involving the scrotum/penis, concomitant STSG can be performed after all tissue has been resected and healthy bleeding edges are achieved, many times during the index operation itself.
The choice of technique is often dependent on surgeon comfort, wound-type, the patient’s fertility goals, and the health of surrounding tissues. At our institution, we tend to utilize STSGs for scrotal reconstruction, as it carries high-success rates in a variety of conditions (38,57,58), and is technically easy to perform. Specifically, when comparing STSG to flaps, skin grafting offers a more natural appearance of the scrotum, provides sub-abdominal temperatures for spermatogenesis, results in minimal post-operative morbidity, and can often be performed in a single stage (59).
Our preference is to utilize the thigh as the donor site for all STSG cases involving only the scrotum and penis. For extensive reconstructions that involve abdominal tissue resection (due to buried penis or lymphedema), we have also had success with utilizing the abdominal skin as a donor site at the area of resection if the skin is not diseased with the primary pathology. We utilize a pneumatic dermatome at a thickness of 0.018 inches and proceed to mesh the graft for all scrotal and abdominal coverage at a ratio of 1.5:1 which allows for relatively small grafts to cover a larger defect (Figure 4). In order to promote the best cosmetic appearance, we tend to avoid meshed grafts for penile wound coverage only. Importantly, we also feel that meshing results in a more natural cosmetic appearance for the scrotum weeks to months following reconstruction (Figures 2-4). Importantly, lubricants such as mineral oil should be spread liberally over the donor site in order to reduce friction prior to dermatome passage. We also stress application of the dermatome at a 45-degree angle to ensure proper and even depth. Before the graft is applied to the scrotal defect, the testes should be sutured together in the midline using 3-0 absorbable suture, and we prefer Vicryl (Ethicon, Inc., Piscataway, NJ, USA); this prevents testicular torsion and allows for the appearance of a bifid, natural-appearing scrotum (Figure 3). If the testes do not come together in the midline, further mobilization of the spermatic cord up to the level of the external ring may be required. Before the testes are covered with the skin graft, we cover the surgical bed with Artiss fibrin sealant which is a longer acting version of Tisseel (Baxter Healthcare, Inc., Deerfield, Il, USA). Artiss allows the surgeon several additional minutes to place the graft and tailor it appropriately prior to the graft being set.
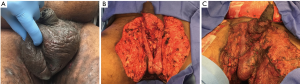
We strongly believe in previously reported data that wound sealants help overall success with graft adherence (60-62). Once the graft has been laid, its edges should be fixated with either skin staples or absorbable sutures, although we exclusively use 3-0 or 4-0 Chromic sutures (Ethicon, Inc., Piscataway, NJ, USA) in an interrupted fashion around the graft edges and have found that quilting stitches can be kept to a minimum following fibrin glue fixation. Traditionally, grafts were held in-place post-operatively with bolstered dressings, commonly using petroleum-based gauze directly over the graft site; however, our preference is to immobilize grafts with VAC devices which stay for a period of 3–5 days based on the severity of the reconstructive repair. The use of negative-pressure dressings has been well-studied and shown to be efficacious at improving graft-take compared to traditional dressings (63,64). During this time period, patients should be on strict bed-rest to prevent disruption of the graft during its most vulnerable period. The donor sites can be coated with mineral oil or antibiotic ointment and covered with Tegaderm dressings (3M, Maplewood, MN, USA) for as long as possible (hopefully days to even weeks). For large donor defects, our preference is to place a closed suction drain underneath the Tegaderm dressing in order to prevent wound seepage. Once the dressings have been removed, patients are instructed to apply antibiotic ointment or Xeroform (Medtronic, Minneapolis, MN, USA) bandaging as needed, bathe as usual, and to keep the area dry if good wound healing has occurred.
In our experience, the most common complication after scrotal skin grafting is wound breakdown, which commonly occurs around the graft edges. In most instances, local wound care with topical agents will provide sufficient treatment. Our patients with non-healing wounds are encouraged to visit our office frequently for wound checks. Additionally, patients can send pictures—securely—for more frequent monitoring of their wounds. Rarely in our experience have patients needed reoperation for wound breakdown after STSG.
Testicular prosthetics
Testicular absence can represent a psychologically traumatic experience in both male children and adults (65). As such, testicular prostheses can be used for a variety of reasons in both children and adults, including testicular torsion, trauma, cancer, atrophy, or transgender reassignment. Though the prosthesis does not provide function, the preservation of male sexual body image is achieved. Testicular prostheses have transformed over the years to today’s current model, which is silicone-coated and saline filled.
Though a variety of testicular prostheses exist, only one is FDA-approved in the United States, the Coloplast Torosa (Coloplast, Minneapolis, MN, USA). Although there are other testicular prostheses available in the European and Asian markets, these are not FDA-approved due to the silicone composition of these devices (66). A 5-year multi-center prospective trial across 18 centers in 1998–1999 assessing the efficacy and safety of the saline-filled testicular prosthesis solidified the FDA’s approval as the current gold-standard testicular prosthesis (67). The Torosa comes in four sizes: extra small, small, medium, and large. Each size corresponds to a fill-volume, and the injectable saline is introduced into the injection port opposite the suture tab. The injection port can only be pierced a total of five times.
Strict aseptic technique is critical during placement of testicular prostheses to prevent infection. A retrospective cohort study out of UCLA in 2002 reported an incidence of 7.3% of patients having a superficial or local wound infection (68). Although there is not one single way to achieve complete sterility, the common routine involves a pre-scrub, perioperative antibiotics, and irrigation within the wound with an antimicrobial solution once the prosthesis is inserted. Most surgeons will change gloves prior to handling the prosthesis. Similar to penile implants, perioperative antibiotics should be geared towards gram-positive, gram-negative, and anaerobic organisms. Bodiwala and colleagues describe conditions used to prevent testicular prosthesis-associated infections, including: sterile urine, pre-operative chlorhexidine shower, perioperative pubic hair shave, systemic and local antibiotics, 10 minute betadine scrub, double gloving, water-proof drapes, and avoiding hematomas (65).
Testicular implants can be placed through a variety of approaches. Surgically implanting a testicular prosthesis can be achieved via a trans-scrotal, high-scrotal, or an inguinal incision. In our adult population, it is our preference to utilize a high scrotal incision for device placement, while some pediatric colleagues tend to perform inguinal approaches due to a higher risk of scrotal erosion (66). Utilization of a high scrotal incision in adults allows for more directed placement and anchoring of the testicular device while also preventing the device from eroding through the scrotum due to the higher location of the incision.
Alternatively, Bush and colleagues describe a technique of placing a testicular prostheses intravaginally at the time of orchiectomy in patients with unsalvageable testes secondary to testicular torsion (69). Although reported with a relatively small cohort (n=12), there were no infectious complications or extrusions at a 5-month median follow up. Furthermore, reported advantages included orthotopic position, extra tunica vaginalis barrier layer, and avoidance of a second anesthetic procedure for placement.
The supra-scrotal or low-inguinal incision is an additional approach commonly used and preferred by pediatric urology colleagues in our region. In this technique, a semilunar incision is made at the junction of the scrotum and the abdominal pubic skin, the intrascrotal space is developed, and the prosthesis is placed. Advocates of this technique report a lower rate of infection as the prosthesis is not in contact with the incision, the pubic hairline can easily hide the incision, and the distance from incision to the scrotum is shorter compared to the inguinal approach (70). Zaontz and colleagues also describe a technique involving the use of a vaginal speculum in placing a prosthesis through an inguinal incision in pediatric patients (71). Placement of testicular prosthesis through an inguinal incision is an easy, ancillary maneuver at the time of orchiectomy, especially for those undergoing orchiectomy for testis cancer. Once orchiectomy has been performed, the scrotum is invaginated, and the prosthesis is sutured into place using permanent suture, taking care to not button-hole the scrotal skin.
Although testicular prostheses have high patient satisfaction, they are not without issues. The most common somatic complications associated with testicular prosthetic surgery are infection (0.6–4%), chronic pain (1–3%), extrusion (3–8%), and hematoma (0.3–3%) (72,73). In terms of dissatisfaction, common complaints include abnormal perceived size of the prosthesis, firmness different than the normal testis, or implant migration to a higher location (73). Regardless, implantation of prostheses is associated with significant improvements in self-esteem using various validated questionnaires (73,74), and can be easily accomplished using a variety of techniques.
Conclusions
We describe several surgical conditions of the scrotum commonly encountered by urologists, ranging from hydrocelectomy to split-thickness skin grafting of the scrotum. Our hope is that the techniques described along with their associated pearls, pitfalls, and complications, will allow more urologists to feel confident in performing these procedures. Below are several key take-home points that summarize our review:
- Early surgical take-back for evacuation of scrotal hematomas is a difficult, but often-necessary decision following scrotal surgery;
- The use of closed-suctions drains for complex scrotal wounds may obviate the need for repeat surgical intervention and we believe this may further help prevent scrotal hematoma formation;
- The management of FG should include, at a minimum, one second-look procedure under anesthesia to ensure adequate debridement of non-viable tissue;
- Wound/graft breakdown of the genitalia can frequently be managed successfully with conservative, local wound-care, and home/office nursing visits;
- In cases with large wound VAC coverage, adherence can be improved with wall-suction, and/or using smaller strips of clear, occlusive dressing;
- STSG adherence can be optimized with the use of a fibrin glue, and often makes placement of the graft technically easier obviating the need for excessive quilting sutures.
Acknowledgements
None.
Footnote
Conflicts of Interest: Jay Simhan is a consultant/advisor for the Coloplast Group and Boston Scientific.
References
- Mihmanli I, Kantarci F, Kulaksizoglu H, et al. Testicular size and vascular resistance before and after hydrocelectomy. AJR Am J Roentgenol 2004;183:1379-85. [Crossref] [PubMed]
- Agrawal MS, Yadav H, Upadhyay A, et al. Sclerotherapy for hydrocele revisited: a prospective randomised study. Indian J Surg 2009;71:23-8. [Crossref] [PubMed]
- Lund L, Kloster A, Cao T. The long-term efficacy of hydrocele treatment with aspiration and sclerotherapy with polidocanol compared to placebo: a prospective, double-blind, randomized study. J Urol 2014;191:1347-50. [Crossref] [PubMed]
- Jahnson S, Sandblom D, Holmang S. A randomized trial comparing 2 doses of polidocanol sclerotherapy for hydrocele or spermatocele. J Urol 2011;186:1319-23. [Crossref] [PubMed]
- Shakiba B, Heidari K, Jamali A, et al. Aspiration and sclerotherapy versus hydrocoelectomy for treating hydrocoeles. Cochrane Database Syst Rev 2014.CD009735. [PubMed]
- Onol SY, Ilbey YO, Onol FF, et al. A novel pull-through technique for the surgical management of idiopathic hydrocele. J Urol 2009;181:1201-5. [Crossref] [PubMed]
- Rowe NE, Martin P, Luke PP. The Western snip, stitch, and tug hydrocelectomy: How I do it. Can Urol Assoc J 2016;10:E328-E330. [Crossref] [PubMed]
- Saber A. New minimally access hydrocelectomy. Urology 2011;77:487-90. [Crossref] [PubMed]
- Rioja J, Sanchez-Margallo FM, Uson J, et al. Adult hydrocele and spermatocele. BJU Int 2011;107:1852-64. [Crossref] [PubMed]
- Swartz MA, Morgan TM, Krieger JN. Complications of scrotal surgery for benign conditions. Urology 2007;69:616-9. [Crossref] [PubMed]
- El-Koutby M. Mohamed Amin el G. Webbed penis: A new classification. J Indian Assoc Pediatr Surg 2010;15:50-2. [Crossref] [PubMed]
- Mondaini N, Ponchietti R, Gontero P, et al. Penile length is normal in most men seeking penile lengthening procedures. Int J Impot Res 2002;14:283-6. [Crossref] [PubMed]
- Deveci S, Martin D, Parker M, et al. Penile length alterations following penile prosthesis surgery. Eur Urol 2007;51:1128-31. [Crossref] [PubMed]
- Wessells H, Lue TF, McAninch JW. Penile length in the flaccid and erect states: guidelines for penile augmentation. J Urol 1996;156:995-7. [Crossref] [PubMed]
- Lee KC, Brock GB. Strategies for maintaining penile size following penile implant. Transl Androl Urol 2013;2:67-73. [PubMed]
- Tran H, Goldfarb R, Ackerman A, et al. Penile Lengthening, Girth, and Size Preservation at the Time of Penile Prosthesis Insertion. Sex Med Rev 2017;5:403-12. [Crossref] [PubMed]
- Alter GJ, Salgado CJ, Chim H. Aesthetic surgery of the male genitalia. Semin Plast Surg 2011;25:189-95. [Crossref] [PubMed]
- Miranda-Sousa A, Keating M, Moreira S, et al. Concomitant ventral phalloplasty during penile implant surgery: a novel procedure that optimizes patient satisfaction and their perception of phallic length after penile implant surgery. J Sex Med 2007;4:1494-9. [Crossref] [PubMed]
- Caso J, Keating M, Miranda-Sousa A, et al. Ventral phalloplasty. Asian J Androl 2008;10:155-7. [Crossref] [PubMed]
- Alter GJ. Correction of penoscrotal web. J Sex Med 2007;4:844-7. [Crossref] [PubMed]
- Chang SJ, Liu SP, Hsieh JT. Correcting penoscrotal web with the V-Y advancement technique. J Sex Med 2008;5:249-50. [Crossref] [PubMed]
- Sorensen MD, Krieger JN, Rivara FP, et al. Fournier’s Gangrene: population based epidemiology and outcomes. J Urol 2009;181:2120-6. [Crossref] [PubMed]
- Eke N. Fournier’s gangrene: a review of 1726 cases. Br J Surg 2000;87:718-28. [Crossref] [PubMed]
- Hagedorn JC, Wessells H. A contemporary update on Fournier’s gangrene. Nat Rev Urol 2017;14:205-14. [Crossref] [PubMed]
- Thwaini A, Khan A, Malik A, et al. Fournier’s gangrene and its emergency management. Postgrad Med J 2006;82:516-9. [Crossref] [PubMed]
- Tahmaz L, Erdemir F, Kibar Y, et al. Fournier’s gangrene: report of thirty-three cases and a review of the literature. Int J Urol 2006;13:960-7. [Crossref] [PubMed]
- Carroll PR, Cattolica EV, Turzan CW, et al. Necrotizing soft-tissue infections of the perineum and genitalia. Etiology and early reconstruction. West J Med 1986;144:174-8. [PubMed]
- Simhan J, Rothman J, Canter D, et al. Gunshot wounds to the scrotum: a large single-institutional 20-year experience. BJU Int 2012;109:1704-7. [Crossref] [PubMed]
- Serkin FB, Soderdahl DW, Hernandez J, et al. Combat urologic trauma in US military overseas contingency operations. J Trauma 2010;69 Suppl 1:S175-8. [Crossref] [PubMed]
- McGeady JB, Breyer BN. Current epidemiology of genitourinary trauma. Urol Clin North Am 2013;40:323-34. [Crossref] [PubMed]
- Michielsen DP, Lafaire C. Management of genital burns: a review. Int J Urol 2010;17:755-8. [Crossref] [PubMed]
- Michielsen D, Van Hee R, Neetens C, et al. Burns to the genitalia and the perineum. J Urol 1998;159:418-9. [Crossref] [PubMed]
- McDougal WS. Lymphedema of the external genitalia. J Urol 2003;170:711-6. [Crossref] [PubMed]
- Garaffa G, Christopher N, Ralph DJ. The management of genital lymphoedema. BJU Int 2008;102:480-4. [Crossref] [PubMed]
- Lymphedema following prostatectomy and radiation therapy. Cancer Pract 1998;6:73-6. [Crossref] [PubMed]
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol 2008;59:596-601. [Crossref] [PubMed]
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009;60:539-61. [Crossref] [PubMed]
- Chen ML, Odom B, Santucci RA. Surgical management of genitoperineal hidradenitis suppurativa in men. Urology 2014;83:1412-7. [Crossref] [PubMed]
- Chawla SN, Gallop C, Mydlo JH. Fournier’s gangrene: an analysis of repeated surgical debridement. Eur Urol 2003;43:572-5. [Crossref] [PubMed]
- Cass AS, Luxenberg M. Testicular injuries. Urology 1991;37:528-30. [Crossref] [PubMed]
- Phonsombat S, Master VA, McAninch JW. Penetrating external genital trauma: a 30-year single institution experience. J Urol 2008;180:192-5; discussion 5-6. [Crossref] [PubMed]
- Mouës CM, van den Bemd GJ, Heule F, et al. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg 2007;60:672-81. [Crossref] [PubMed]
- Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553-62. [Crossref] [PubMed]
- Assenza M, Cozza V, Sacco E, et al. VAC (Vacuum Assisted Closure) treatment in Fournier's gangrene: personal experience and literature review. Clin Ter 2011;162:e1-5. [PubMed]
- Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am J Surg 2009;197:660-5; discussion 5. [Crossref] [PubMed]
- Morey AF, Brandes S, Dugi DD 3rd, et al. Urotrauma: AUA guideline. J Urol 2014;192:327-35. [Crossref] [PubMed]
- Buckley JC, McAninch JW. Use of ultrasonography for the diagnosis of testicular injuries in blunt scrotal trauma. J Urol 2006;175:175-8. [Crossref] [PubMed]
- Quarmby CJ, Millar AJ, Rode H. The use of diverting colostomies in paediatric peri-anal burns. Burns 1999;25:645-50. [Crossref] [PubMed]
- Peck MD, Boileau MA, Grube BJ, et al. The management of burns to the perineum and genitals. J Burn Care Rehabil 1990;11:54-6. [Crossref] [PubMed]
- Kagan RJ, Yakuboff KP, Warner P, et al. Surgical treatment of hidradenitis suppurativa: a 10-year experience. Surgery 2005;138:734-40; discussion 40-1. [Crossref] [PubMed]
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016;375:422-34. [Crossref] [PubMed]
- Bickell M, Beilan J, Wallen J, et al. Advances in Surgical Reconstructive Techniques in the Management of Penile, Urethral, and Scrotal Cancer. Urol Clin North Am 2016;43:545-59. [Crossref] [PubMed]
- Mopuri N, O'Connor EF, Iwuagwu FC. Scrotal reconstruction with modified pudendal thigh flaps. J Plast Reconstr Aesthet Surg 2016;69:278-83. [Crossref] [PubMed]
- Chen SY, Fu JP, Chen TM, et al. Reconstruction of scrotal and perineal defects in Fournier's gangrene. J Plast Reconstr Aesthet Surg 2011;64:528-34. [Crossref] [PubMed]
- Yao H, Zheng D, Wen J, et al. Reconstruction of major scrotal defects by anterolateral thigh flap. Cell Biochem Biophys 2014;70:1331-5. [Crossref] [PubMed]
- Karian LS, Chung SY, Lee ES. Reconstruction of Defects After Fournier Gangrene: A Systematic Review. Eplasty 2015;15:e18. [PubMed]
- Alwaal A, McAninch JW, Harris CR, et al. Utilities of Split-Thickness Skin Grafting for Male Genital Reconstruction. Urology 2015;86:835-9. [Crossref] [PubMed]
- Morey AF, Meng MV, McAninch JW. Skin graft reconstruction of chronic genital lymphedema. Urology 1997;50:423-6. [Crossref] [PubMed]
- Maguiña P, Palmieri TL, Greenhalgh DG. Split thickness skin grafting for recreation of the scrotum following Fournier's gangrene. Burns 2003;29:857-62. [Crossref] [PubMed]
- Boccara D, De Runz A, Bakara F, et al. Artiss Sealant(R): An Alternative to Stapling Skin Grafts on the Dorsal Side of the Hand and Fingers. J Burn Care Res 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Branski LK, Mittermayr R, Herndon DN, et al. Fibrin sealant improves graft adherence in a porcine full-thickness burn wound model. Burns 2011;37:1360-6. [Crossref] [PubMed]
- Gibran N, Luterman A, Herndon D, et al. Comparison of fibrin sealant and staples for attaching split-thickness autologous sheet grafts in patients with deep partial- or full-thickness burn wounds: a phase 1/2 clinical study. J Burn Care Res 2007;28:401-8. [Crossref] [PubMed]
- Azzopardi EA, Boyce DE, Dickson WA, et al. Application of topical negative pressure (vacuum-assisted closure) to split-thickness skin grafts: a structured evidence-based review. Ann Plast Surg 2013;70:23-9. [Crossref] [PubMed]
- Petkar KS, Dhanraj P, Kingsly PM, et al. A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split-thickness skin grafts in burned patients. Burns 2011;37:925-9. [Crossref] [PubMed]
- Bodiwala D, Summerton DJ, Terry TR. Testicular prostheses: development and modern usage. Ann R Coll Surg Engl 2007;89:349-53. [Crossref] [PubMed]
- Kogan S. The clinical utility of testicular prosthesis placement in children with genital and testicular disorders. Transl Androl Urol 2014;3:391-7. [PubMed]
- Turek PJ, Master VA. Testicular Prosthesis Study G. Safety and effectiveness of a new saline filled testicular prosthesis. J Urol 2004;172:1427-30. [Crossref] [PubMed]
- Mohammed A, Yassin M, Hendry D, et al. Contemporary practice of testicular prosthesis insertion. Arab J Urol 2015;13:282-6. [Crossref] [PubMed]
- Bush NC, Bagrodia A. Initial results for combined orchiectomy and prosthesis exchange for unsalvageable testicular torsion in adolescents: description of intravaginal prosthesis placement at orchiectomy. J Urol 2012;188:1424-8. [Crossref] [PubMed]
- Libman JL, Pippi-Salle JL, Chan PT. The use of a suprascrotal or “wink” incision for placing a testicular prosthesis. BJU Int 2006;98:1051-3. [Crossref] [PubMed]
- Zaontz MR, Ritchie EL, Maizels M, et al. Insertion of testicular prosthesis: use of vaginal speculum. Urology 1990;35:130-2. [Crossref] [PubMed]
- Marshall S. Potential problems with testicular prostheses. Urology 1986;28:388-90. [Crossref] [PubMed]
- Catanzariti F, Polito B, Polito M. Testicular prosthesis: Patient satisfaction and sexual dysfunctions in testis cancer survivors. Arch Ital Urol Androl 2016;88:186-8. [Crossref] [PubMed]
- Yossepowitch O, Aviv D, Wainchwaig L, et al. Testicular prostheses for testis cancer survivors: patient perspectives and predictors of long-term satisfaction. J Urol 2011;186:2249-52. [Crossref] [PubMed]


