Is contrast enhancement needed for diagnostic prostate MRI?
Introduction
Recent Prostate Imaging Reporting and Data System version 2 (PI-RADS v2) using multiparametric magnetic resonance imaging (mpMRI) [T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)], established the guidelines to promote global standardization of prostate imaging, to improve detection, localization, characterization and risk stratification of prostate cancer (PCa) as well as to improve communication with referring urologists (1).
PI-RADS v2 determines the role of each sequence (T2WI, DWI and DCE) and, by combining and assessing on a 5 point category scale the findings on each sequence, correlates with the presence of clinically significant PCa and its localization.
Among potential ambiguities and gaps (2), an unclear guidance on clinical management for each one of the five categories has emerged. Particularly, the PI-RADS score 3 is insufficient for decision-making (biopsy or not biopsy) and the positive DCE upgrading this category to a score 4 is irrelevant (3,4).
In this scenario, some Authors have recently proposed a standardization and simplification of PI-RADS v2 using the bpMRI approach (5-7). Diagnosis of PCa is histological. BpMRI typically meets the objective of detection and localization of PCa suspected foci as well as the execution of MRI-targeted or MRI-TRUS-guided fusion prostate biopsy. Depiction of index lesion and its volume by bpMRI could represent a potential alternative tool to determine PCa presence and severity, to guide patient’s management and to defeat the use of contrast material in patients suspected of PCa.
We here reviewed the role of DCE-MRI. The potential contributes of bpMRI (T2WI and DWI) and lesion volume in the diagnosis and management of suspected PCa were reported as well.
Current state of MRI sequences in the diagnosis of PCa
Despite the detailed information provided by the American College of Radiology (ACR) (8), the working group of the European Society of Urogenital Radiology (ESUR) (9) and the latest version of PI-RADS 2.0 (1), the “gold standard” for evaluating PCa aggressiveness is the Gleason score (GS), measured after prostate biopsy or radical prostatectomy.
Since the correlation between mpMRI and PCa aggressiveness remains controversial (1), the main objective of mpMRI is the detection and localization of suspicious PCa.
In the PI-RADS v2 spectroscopy has been omitted and DCE has assumed a minor role; DWI and T2WI are respectively considered the predominant sequences for lesion detection in peripheral zone (PZ) and transitional zone (TZ) (1). T2WI and DWI seem to be sufficient for co-registration of MRI-TRUS images and then for guidance of subsequent biopsy of suspicious lesions identified by bpMRI.
DCE-MRI potential and drawbacks
According to PI-RADS v2, a lesion in PZ assigned to PI-RADS 3 score is upgraded to PI-RADS 4 when it shows enhancement by DCE (1). Since the addition of DCE does not actually seem to alter the clinical implications (7), the role of T2WI and DWI has been emphasized. DCE may potentially be useful:
- to help the detection of some subtle tumors which, due to small size, poor visual contrast, compared to adjacent benign tissue, or a challenging location within prostate, tend to be missed using the combination of T2WI and DWI alone;
- to aid imaging when imaging quality of T2WI and DWI is impaired;
- to assist the scoring level of suspicion for clinical significant cancer in case of equivocal lesions;
- to assess recurrent disease in patients who received treatment (2). DCE-MRI can monitor the response to treatment and increase the diagnostic accuracy of locoregional recurrence in patients who underwent radical prostatectomy (sensitivity 46–90%; specificity 74–96%) (10). Moreover, it has been suggested as a promising biomarker for assessing and predicting therapeutic response in PCa providing information about the action of therapeutics and potentially helping to distinguish responders from non-responders (11);
- to assess tumor microvascularization and angiogenesis (11). On DCE-MRI, PCa shows earlier and stronger enhancement of vessels than surrounding normal prostate tissue does (12).
Contrary to the above mentioned potential values, DCE in PCa exhibits the following defective features:
- DCE is a not-specific sequence to detect PCa in the peripheral, transitional and central zone and to determine tumor aggressiveness correlated with the type of enhancement curve. Moreover, it is often difficult to differentiate small areas of enhancement, especially in the transitional area, from the adjacent normal prostate tissue (10);
- DCE sequences and curve type play a secondary role in the accurate determination of lesion category using PI-RADS providing the assessment of a positive or negative score (1);
- the enhancement curves are not-specific and have low sensitivity in identifying suspicious PCa foci. Therefore, the role of the DCE should ensure the enhancement of suspicious lesions identified on T2WI or DWI, compared to the surrounding glandular tissue (10);
- DCE score depends on variability in reader interpretations, mainly due to ambiguity in the definition of positive and negative enhancement (2);
- it is moreover not clear how to consider a multifocal early background enhancement, while diffuse enhancement is considered a negative finding. As a consequence, a future improvement of PI-RADS v2 should be necessary to overcome weaknesses about technical aspects of the acquisition, postprocessing and interpretation of DCE (2);
- the optimum imaging period after gadolinium injection remains unknown, with subsequent lack of standardization in data acquisition protocols.
DCE additionally displays limits concerning both cost and time necessary to complete the study which are related to the use of gadolinium-based contrast agents. The potential risks of these contrast materials in patients with a glomerular filtration <30 mL/min and their accumulation in the brain have to be considered (13,14).
Biparametric MRI (bpMRI) protocol and clinical results
At our institution, MRI of the prostate is performed on a 3 T scanner (Achieva, Philips Medical Systems, Healthcare, Eindhoven, the Netherlands) without an endorectal coil. The bpMRI protocol consists of axial T1W gradient-echo sequence with fat-suppression technique (THRIVE) imaging, axial, sagittal and coronal T2W FSE imaging, axial DWI sequence with B values of 0, 750, 1,500 and 2,000 s/mm2 and apparent diffusion coefficient (ADC) maps calculation.
On bpMRI data we calculated volumes of both prostate and index lesion using the ellipsoidal formula (maximum anterior posterior diameter × maximum transverse diameter × maximum longitudinal diameter × 0.52) and/or by a software with 3D reconstruction. A freehand region of interest (ROI) around the seminal vesicles, TZ and PZ of the prostate and urethra, from base to the apex is drawn and then around the index lesion; the software reproduced, automatically and in real-time, 3D volumetric graphic their representation in different transparent colors after applied translation and rotation. In the meanwhile, the volume values, expressed in cm3, were automatically calculated and registered.
The knowledge of the value of the individual sequences is essential in the detection, localization and management of suspicious PCa.
Several studies demonstrate that DCE has a limited contribution to the information provided by T2WI and DWI alone or in addition to T2WI and DWI (15-22) and with reported sensitivities and specificities of 71–84% and 33–79%, respectively, for PCa of any grade, and 80–90% and 47–86% for high-grade PCa (2,15,17,18,23-32).
The diagnostic value of bpMRI in men with or without prior biopsy and combined with prostate-specific antigen (PSA) has been validated, resulting an improved accuracy for detecting clinically significant PCa and to direct biopsy needles under TRUS guidance, after MRI-ultrasonography fusion (6,15,33-36). Recently, De Visschere et al. (7) demonstrated that the role of DCE is limited for diagnosis of clinically significant PCa in patients with elevated PSA before biopsy and that DCE should be reserved only to those patients with a score 3 lesion on DWI in PZ and no suspicious lesion in TZ. The authors demonstrated that DCE was redundant in 80.8% of patients while in the 19.2% of patients, the supplementary information (enhancement or not) was incorrect in approximately 30% resulting contrast medium not necessary in the vast majority of patients when using PI-RADS v2; in the remaining 19.2% of patients, the additional information (enhancement or not) was incorrect in approximately 30% of the cases, resulting in an unnecessary use of DCE in the majority of patients evaluated by PI-RADS v2.
In accordance with previous reports, in our experience we found that bpMRI and mpMRI have similar diagnostic accuracy in the detection of PCa index lesions (Figure 1).
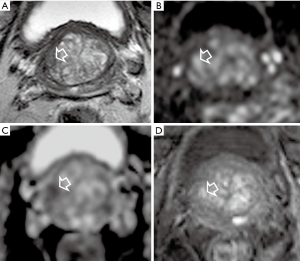
For our study we examined patients with PSA abnormalities, with or without previous negative biopsies, submitted to radical prostatectomy. The histological findings were considered as standard reference (37).
For the index lesion detection, we measured the diagnostic performance of T2WI, DWI and DCE MRI alone or combined in bpMRI (T2WI and DWI) or mpMRI (T2WI, DWI and DCE). Compared to the standard reference, the sensitivity of DWI, T2WI and DCE, alone, was in the order: DWI > T2WI > DCE. For DWI, it was 97.6%, 100% and 94.7% in the whole prostate or for PZ and TZ, respectively. The sensitivity of T2WI and of DCE-MRI was low, assuming values of 68.3%, 47.4% and 86.4% or of 39.02%, 31.6% and 45.4% for the whole prostate, TZ and PZ, respectively. Both types of combined MRI (bpMRI and mpMRI) exhibited same level of sensitivity which corresponded to DWI value (100% in PZ, 97.6% and 94.7% in the entire prostate or TZ, respectively). Analogous trends were observed by assessing the agreement (measured by Cohen’s k coefficient) between the MRI approaches and the reference standard. As for sensitivity, the agreement of bpMRI and mpMRI (which corresponded to the value of DWI alone) were identical, indicating that DCE sequence in mpMRI did not contribute to index lesion detection in PZ and in TZ (37). Evaluating both index and non-index lesions by bpMRI, we found 27.6% false-positives and 3.3% false-negatives. However, the diagnostic performance of bpMRI increased with the size of lesion and, interestingly, assumed high values for lesions ≥10 mm, which can only mean a reduced risk of both false-positives and false-negative, for bpMRI, in the group of the clinically significant lesions (GS ≥7) (37).
bpMRI and lesion volume as alternative to multiparametric MRI
Although DCE-MRI quantitative parameters have the potential to assess PCa aggressiveness (low grade from intermediate and high grade) in PZ (38), to date no definitive studies have been reported about the correlation between DCE and cancer aggressiveness. MRI has the greatest potential to depict volumes in clinical relevant lesions (2,39-49), and several studies have reported a sufficiently reliable correspondence between volume of the suspicious lesion (measured on T2WI and DWI) and tumor volume measured at histological examination of the prostate (50-52).
In this perspective, the bpMRI could be considered a reliable tool for estimating volume of PCa as in the case of other solid tumors.
PCa is considered a clinically significant neoplasia if Gleason score is ≥7 and tumor volume >0.5 mL (53). According to the findings mentioned above, the volume of the suspicious lesion may have a discriminating role in the management of the patients with MRI evidence of suspicious lesion at risk for PCa. Since the most effective way to reduce overtreatment of PCa is to limit its detection in men with low-risk disease, assessment of the aggressiveness of the PCa is crucial in identifying appropriate patient-tailored management.
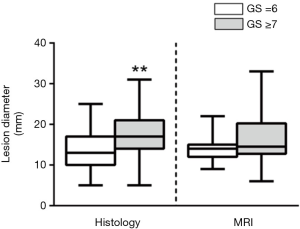
In clinical practice, when we take into the account an appropriate decision-making (biopsy or active surveillance), the main weakness emerging from the PI-RADS v2 is the lesion diameter. According to PI-RADS v2, a lesion with a PI-RADS score of 4 is upgraded to the higher score when its diameter is >15 mm (1). To our knowledge, no studies demonstrated a relationship between the lesion diameter and cancer aggressiveness. Additionally, in our recent experience we found that lesion diameter detected by MRI seems not to be able to predict tumor aggressiveness. In contrast with histology, indeed, no statistical differences of tumor lesion diameter between GS =6 and GS ≥7 groups (37) were seen (Figure 2).
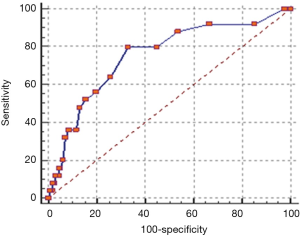
PCa often is a solid tumor with a defined three dimensional shape. Our recent data demonstrated the potential diagnostic power of MRI lesion volume for PCa and tumor aggressiveness (differentiation of clinically significant cancer >0.5 versus <0.5 mL) (52). Detection of aggressive PCa (GS ≥7) increased with the increase of lesion volume by 13.8% to 40% or to 55% considering a lesion volume <0.5 mL or between 0.5 and 1 mL or >1 mL, respectively (P=0.008). In addition, the area under the ROC curve (performed by comparing values of tumor lesion volume in GS ≥7 and GS =6 groups) was 0.752 (P<0.0001) and the cut-off lesion volume was >0.5 mL with a sensitivity and specificity of 80% and 60% respectively (52) (Figure 3).
Moreover, although the gold standard for assessment of PCa clinical significance are the Gleason score and tumor volume, obtained from prostate biopsy and/or radical prostatectomy specimens, several studies demonstrated that MRI has the greatest potential to depict volumes in clinical relevant lesions (50-52).
Hovewer, the lesion volume has some limits: (I) volume calculation of suspected lesion requires the use of an appropriate software (because estimation with the ellipsoid model might be too rough); (II) although there are several studies that show a correlation between volume of neoplasia (measured at the final histological examination) and volume of suspected lesion (measured with the appropriate software), this evidence is not definitive (multicenter, prospective studies, etc.).
A new risk stratification system as alternative to PI-RADS v2: clinical management and lesion categories correlation
PI-RADS v2 score does not offer a clear guidance on the clinical management for each one of the five categories. In particular, a consensus was reached regarding the need of not performing the biopsy for score 1 and 2 lesion and to perform biopsy for score 4 and 5 lesion. Conversely, there is still no consensus for the management of PI-RADS 3 lesions (equivocal for the presence of clinical significant cancer and/or indeterminate for biopsy). DCE plays a limited role and is ignored in TZ and in PZ, except when DWI has been assigned a score, then the overall assessment category may be upgraded to a score 4 of a positive DCE that in many cases may be result redundant. As a consequence, recently an alternative overall assessment score by T2WI and DWI yielded similar performance as PI-RADS v2 (7).
According to the criteria and lexicon of the PI-RADS v2 guidelines (1), in our experience, the image analysis was based on the recognition of lesion pattern on DWI (lesion hyperintense) and ADC map (lesion moderately/markedly hypointense), at first, and on T2WI (lesion moderately hypointense/hypointense) sequences, later. DWI, with high b values, together with ADC images represented the predominant sequence to detect the lesion both in PZ and TZ.
Although the most important limitation for the adoption of bpMRI in clinical practice is the lack of a standardized scoring system for risk assessment of suspicious lesions, in our experience, the bpMRI and lesion volume calculation represent potential tools to manage suspected PCa (biopsy in >0.5 mL volume and <0.5 mL volume or no biopsy), in particular in PI-RADS 3 lesions.
Representative cases of bpMRI and lesion volume in patients with suspicious PCa are reported in Figures 4-6.
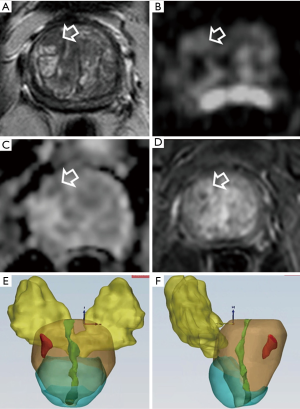
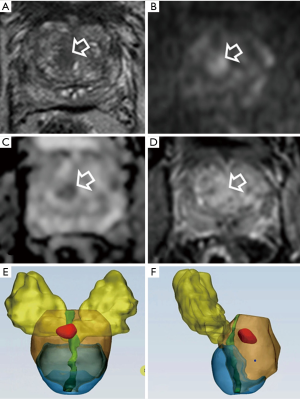
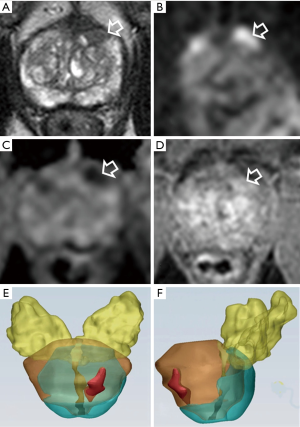
According to others (7), omitting DCE in all patients seems reasonable in the clinical situation where prostate MRI is used as a method of risk stratification of clinical significant PCa in patients with elevated PSA. In addition, bpMRI reveals to be a valid tool in the detection of local recurrence after prostatectomy.
In our experience bpMRI (DWI sequences and ADC maps in association with T2WI), without endorectal coil, as an alternative approach to mpMRI examination, is justified (I) to identify and localize lesions in the PZ, TZ and in the anterior fibromuscular stroma; (II) by no use of gadolinium-based contrast agent; (III) by reduction of examination time and costs.
A comparative study between mpMRI and bpMRI and volume of the lesion measurement is underway. Our preliminary data show a higher predictive value of lesion volume compared to DCE in terms of overall cancer detection rate and cancer aggressiveness. In this perspective, the bpMRI and lesion volume index calculation, result more appropriate than mpMRI for cancer diagnosis and clinical decision making (biopsy or not biopsy) in patients suspected of having PCa.
Conclusions
In clinical practice, the development of imaging techniques, examination protocols and 3D software improved diagnosis of usual and unusual malignancies (54-56). Particularly, mpMRI modified the approach to the patient with suspicious of PCa and became an useful tool to detect clinical significant cancer. However, gadolinium, long test times and higher costs represent limits for mpMRI. By demonstrating similar diagnostic accuracy of bpMRI and mpMRI in the detection of PCa, gadolinium-based contrast agent seems not to be strictly necessary in the detection and localization of PCa, and bpMRI and lesion volume calculation are sufficient for these purposes. We emphasize the clinical use of the bpMRI considering the use of no gadolinium, reduction of the costs and time required to complete the study.
Acknowledgements
We thank Marco Piergentini and Giacomo Costantini for their valuable contribution to 3D reconstructions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Prostate Imaging Reporting and Data System (PI-RADS). Reston (VA): American College of Radiology. Available online: http://www.acr.org/Quality-Safety/ Resources/PIRADS/
- Rosenkrantz AB, Oto A, Turkbey B, et al. Prostate Imaging Reporting and Data System (PI-RADS), Version 2: A Critical Look. AJR Am J Roentgenol 2016;206:1179-83. [Crossref] [PubMed]
- Abd-Alazeez M, Kirkham A, Ahmed HU, et al. Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies as the reference standard. Prostate Cancer Prostatic Dis 2014;17:40-6. [Crossref] [PubMed]
- Renard-Penna R, Roupret M, Compérat E, et al. Relationship between non-suspicious MRI and insignificant prostate cancer: results from a monocentric study. World J Urol 2016;34:673-8. [Crossref] [PubMed]
- Scialpi M, Falcone G, Scialpi P, et al. Biparametric MRI: a further improvement to PIRADS 2.0? Diagn Interv Radiol 2016;22:297-8. [Crossref] [PubMed]
- Scialpi M, Martorana E, D’Andrea A. Standardizing Biparametric MRI to Simplify and Improve Prostate Imaging Reporting and Data System, Version 2, in Prostate Cancer Management. AJR Am J Roentgenol 2016.W1-2. [PubMed]
- De Visschere P, Lumen N, Ost P, et al. Dynamic contrast-enhanced imaging has limited added value over T2-weighted imaging and diffusion-weighted imaging when using PI-RADSv2 for diagnosis of clinically significant prostate cancer in patients with elevated PSA. Clin Radiol 2017;72:23-32. [Crossref] [PubMed]
- Eberhardt SC, Carter S, Casalino DD, et al. ACR Appropriateness Criteria prostate cancer—pretreatment detection, staging, and surveillance. J Am Coll Radiol 2013;10:83-92. [Crossref] [PubMed]
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- Verma S, Turkbey B, Muradyan N, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol 2012;198:1277-88. [Crossref] [PubMed]
- Fennessy FM, McKay RR, Beard CJ, et al. Dynamic contrast-enhanced magnetic resonance imaging in prostate cancer clinical trials: potential roles and possible pitfalls. Transl Oncol 2014;7:120-9. [Crossref] [PubMed]
- Engelbrecht MR, Huisman HJ, Laheij RJ, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology 2003;229:248-54. [Crossref] [PubMed]
- Beomonte Zobel B, Quattrocchi CC, Errante Y, et al. Gadolinium-based contrast agents: did we miss something in the last 25 years? Radiol Med 2016;121:478-81. [Crossref] [PubMed]
- Olchowy C, Cebulski K, Łasecki M, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity—A systematic review. PLoS One 2017;12:e0171704. [Crossref] [PubMed]
- Delongchamps NB, Rouanne M, Flam T, et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int 2011;107:1411-8. [Crossref] [PubMed]
- Vargas HA, Hötker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 2016;26:1606-12. [Crossref] [PubMed]
- de Rooij M, Hamoen EH, Fütterer JJ, et al. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014;202:343-51. [Crossref] [PubMed]
- Roethke MC, Kuru TH, Schultze S, et al. Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 Tesla. Eur Radiol 2014;24:344-52. [Crossref] [PubMed]
- Weinreb JC, Blume JD, Coakley FV, et al. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy--results of ACRIN prospective multi-institutional clinicopathologic study. Radiology 2009;251:122-33. [Crossref] [PubMed]
- Vargas HA, Akin O, Shukla-Dave A, et al. Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology 2012;265:478-87. [Crossref] [PubMed]
- Tan CH, Hobbs BP, Wei W, et al. Dynamic contrast-enhanced MRI for the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol 2015;204:W439-48. [Crossref] [PubMed]
- Haghighi M, Shah S, Taneja SS, et al. Prostate cancer: diffusion-weighted imaging versus dynamic-contrast enhanced imaging for tumor localization-a meta-analysis. J Comput Assist Tomogr 2013;37:980-8. [Crossref] [PubMed]
- Thompson JE, Moses D, Shnier R, et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study. J Urol 2014;192:67-74. [Crossref] [PubMed]
- Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol 2006;50:1163-74; discussion 1175. [Crossref] [PubMed]
- Arumainayagam N, Ahmed HU, Moore CM, et al. Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology 2013;268:761-9. [Crossref] [PubMed]
- Kumar V, Jagannathan NR, Thulkar S, et al. Prebiopsy magnetic resonance spectroscopy and imaging in the diagnosis of prostate cancer. Int J Urol 2012;19:602-13. [Crossref] [PubMed]
- Villeirs GM, Oosterlinck W, Vanherreweghe E, et al. A qualitative approach to combined magnetic resonance imaging and spectroscopy in the diagnosis of prostate cancer. Eur J Radiol 2010;73:352-6. [Crossref] [PubMed]
- Portalez D, Mozer P, Cornud F, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol 2012;62:986-96. [Crossref] [PubMed]
- Kuru TH, Roethke MC, Rieker P, et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardised scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU Int 2013;112:1080-7. [Crossref] [PubMed]
- Schimmöller L, Quentin M, Arsov C, et al. Inter-reader agreement of the ESUR score for prostate MRI using in-bore MRI-guided biopsies as the reference standard. Eur Radiol 2013;23:3185-90. [Crossref] [PubMed]
- Hamoen EH, de Rooij M, Witjes JA, et al. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol 2015;67:1112-21. [Crossref] [PubMed]
- Junker D, Schäfer G, Edlinger M, et al. Evaluation of the PI-RADS scoring system for classifying mpMRI findings in men with suspicion of prostate cancer. Biomed Res Int 2013;2013:252939. [Crossref] [PubMed]
- Rais-Bahrami S, Siddiqui MM, Vourganti S, et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int 2015;115:381-8. [Crossref] [PubMed]
- Radtke JP, Boxler S, Kuru TH, et al. Improved detection of anterior fibromuscular stroma and transition zone prostate cancer using biparametric and multiparametric MRI with MRI-targeted biopsy and MRI-US fusion guidance. Prostate Cancer Prostatic Dis 2015;18:288-96. [Crossref] [PubMed]
- Sertkaya Z. Re: Fascelli et al: Combined Biparametric Prostate Magnetic Resonance Imaging and Prostate-specific Antigen in the Detection of Prostate Cancer: A Validation Study in a Biopsy-naive Patient Population (Urology 2016;88:125-134). Urology 2016;95:223. [Crossref] [PubMed]
- Thestrup KC, Logager V, Baslev I, et al. Biparametric versus multiparametric MRI in the diagnosis of prostate cancer. Acta Radiol Open 2016;5:2058460116663046. [PubMed]
- Scialpi M, Prosperi E, D'Andrea A, et al. Biparametric versus Multiparametric MRI with Non-endorectal Coil at 3T in the Detection and Localization of Prostate Cancer. Anticancer Res 2017;37:1263-71. [Crossref] [PubMed]
- Vos EK, Litjens GJ, Kobus T, et al. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3 T. Eur Urol 2013;64:448-55. [Crossref] [PubMed]
- Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011;186:1818-24. [Crossref] [PubMed]
- Villers A, Lemaitre L, Haffner J, et al. Current status of MRI for the diagnosis, staging and prognosis of prostate cancer: implications for focal therapy and active surveillance. Curr Opin Urol 2009;19:274-82. [Crossref] [PubMed]
- Verma S, Rajesh A, Morales H, et al. Assessment of aggressiveness of prostate cancer: correlation of apparent diffusion coefficient with histologic grade after radical prostatectomy. AJR Am J Roentgenol 2011;196:374-81. [Crossref] [PubMed]
- Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 2011;259:775-84. [Crossref] [PubMed]
- Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 2011;258:488-95. [Crossref] [PubMed]
- Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011;259:453-61. [Crossref] [PubMed]
- Peng Y, Jiang Y, Yang C, et al. Quantitative analysis of multiparametric prostate MR images: differentiation between prostate cancer and normal tissue and correlation with Gleason score--a computer-aided diagnosis development study. Radiology 2013;267:787-96. [Crossref] [PubMed]
- Donati OF, Afaq A, Vargas HA, et al. Prostate MRI: evaluating tumor volume and apparent diffusion coefficient as surrogate biomarkers for predicting tumor Gleason score. Clin Cancer Res 2014;20:3705-11. [Crossref] [PubMed]
- Donati OF, Mazaheri Y, Afaq A, et al. Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology 2014;271:143-52. [Crossref] [PubMed]
- Isebaert S, Van den Bergh L, Haustermans K, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging 2013;37:1392-401. [Crossref] [PubMed]
- Mazaheri Y, Hricak H, Fine SW, et al. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology 2009;252:449-57. [Crossref] [PubMed]
- Radtke JP, Schwab C, Wolf MB, et al. Multiparametric Magnetic Resonance Imaging (MRI) and MRI-Transrectal Ultrasound Fusion Biopsy for Index Tumor Detection: Correlation with Radical Prostatectomy Specimen. Eur Urol 2016;70:846-53. [Crossref] [PubMed]
- Baco E, Ukimura O, Rud E, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol 2015;67:787-94. [Crossref] [PubMed]
- Martorana E, Pirola GM, Scialpi M, et al. Lesion volume predicts prostate cancer risk and aggressiveness: validation of its value alone and matched with prostate imaging reporting and data system score. BJU Int 2017;120:92-103. [PubMed]
- Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993;71:933-8. [Crossref] [PubMed]
- Scialpi M, Cagini L, Pierotti L, et al. Detection of small (≤2 cm) pancreatic adenocarcinoma and surrounding parenchyma: correlations between enhancement patterns at triphasic MDCT and histologic features. BMC Gastroenterol 2014;14:16. [Crossref] [PubMed]
- Scialpi M, Roncati L, Pusiol T. Multiparametric MRI at 3T of Usual Prostatic Carcinoma with Neuroendocrine Differentiation First Case Report. Erciyes Med J 2017;39:42-3. [Crossref]
- Manganaro L, Vinci V, Giancotti A, et al. Bi-parametric magnetic resonance imaging applied to obstetrics. J Obstet Gynaecol 2017;37:670-2. [PubMed]


