Impact of bladder dysfunction in the management of post radical prostatectomy stress urinary incontinence—a review
Introduction
Radical prostatectomy (RP) is the treatment of choice for patients with localized prostate cancer. Despite advances in pelvic anatomy and surgical technique, the overall incidence of post RP incontinence continues to rise due to the increasing numbers of RP performed (1,2). Currently the prevalence of post-prostatectomy incontinence (PPI) varies from 1% to 87%, depending on the definition, timing of evaluation, surgical approach and who carries out the assessment (3-5). PPI is multi-factorial and is due to intrinsic sphincter deficiency (ISD) and to pre-existing bladder dysfunction or dysfunction arising de novo post RP (6-8). Table 1 shows studies reporting the cause of PPI. ISD is considered to be the most important and most common contributing factor to PPI; however detrusor overactivity (DO), detrusor underactivity (DU) and poor bladder compliance commonly occur with ISD or in isolation, and are important factors in PPI (12).
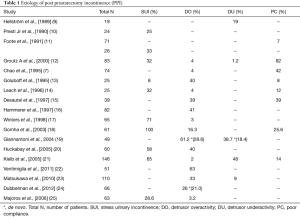
Full table
The mainstay of treatment of PPI due to ISD is the insertion of an artificial urinary sphincter (AUS), and male slings. There has been some experience with transurethral injection of bulking agent but generally this is offered only to patients with mild ISD. There are no control trials comparing patients with PPI undergoing urodynamic studies (UDS) vs. no UDS prior to AUS insertion. Some papers suggest that the presence of bladder dysfunction does not alter post AUS continence outcomes (26-29). It has also been demonstrated that bladder dysfunction may improve after AUS implantation (30). Nevertheless, AUS placement in those with reduced compliance may lead to upper tract damage (31). Performing UDS has its advantages as it allows the clinician to assess, treat and counsel those with concurrent bladder dysfunction. If severe bladder dysfunction is identified, treatment of presumptive SUI and its potential complications may be avoided. This can lead to improved quality of life and prevention of complications especially when concurrent treatments of bladder dysfunction may potentially compromise each other.
This review seeks to report the etiology, evaluation, and management of non-urethral post-prostatectomy incontinence. The impact of bladder dysfunction on stress urinary incontinence (SUI) management is also explored. An effort has been made to provide an algorithm to clinicians for appropriate surgical management. The surgical techniques of commonly performed procedures and their outcomes are described.
Methods
A comprehensive literature inquiry using the following medical search engines were performed; PubMed, Ovid, Science Direct and Google Scholar. The search included a combination of the following terms: post-prostatectomy incontinence, DO, DU, impaired compliance, anticholinergic, onabotulinumtoxinA and sacral neuromodulation (SNM). Search results were assessed for their overall relevance to this review. Definitions, general overview and management options were extracted from the relevant medical literature.
Pathophysiology of incontinence post PR
The majority of PPI results from ISD which is due to injury to the rhabdosphincter during the apical dissection and denervation of to the neurovascular bundles during RP (6). Bladder dysfunction, such as DO, DU and impaired bladder compliance can be present before RP, or may arise due to the surgery. Mobilization of the bladder can result in partial autonomic and somatic decentralization as well as inflammation, infection, bladder wall alterations and hypoxia (12,31,32).
Pre-operative DO can be due to bladder outlet obstruction (BOO) from an enlarged prostate. De novo DO may be secondary to BOO from bladder neck contracture or urethral strictures. It is also postulated that DO results from urethral afferent activity when SUI is present. This is believed to be the basis for the reversibility of bladder dysfunction when SUI is successfully treated. Denervation injury to the bladder is the main cause of DU. As for impaired bladder compliance, pelvic surgery such as RP with or without adjuvant radiation therapy can result in bladder fibrosis and contracture, affecting compliance negatively (32).
Post PR detrusor overactivity
Incidence of post prostatectomy DO
DO has been reported to occur at extremely varying rates between 2% and 63% post RP. Kielb et al., found that in patients with PPI, only 2% had DO (21). Similarly, Majoros et al., found DO in 3.2% of 63 patients with PPI and Winters et al., found DO to be the sole cause of PPI in 3.3% (17,25). Huckabay et al., and Groutz et al., reported that PPI was due to DO in 13% and 7.2% of patients respectively (12,20). Differing from these findings, Ventimiglia et al., found DO in 63% with PPI 8–24 months post nerve-sparing RP and considered incontinence to be purely due to DO in 35% of patients (33). Likewise, Leach et al., established that DO contributed to incontinence in 60% of patients (14). DO frequently occurs with other bladder dysfunction post RP. Chao et al., found that only 4% of 74 patients with incontinence after prostatectomy had DO alone, while 39% had mixed bladder and sphincter dysfunction (7). Matsukawa et al., found in patients who underwent UDS before and after laparoscopic RP (LRP), a DO rate of 33% in addition to a DU rate of 9% (23).
Curiously, RP can affect pre-existing DO in different ways. Constantinou et al., showed that in patients with pre-existing DO, RP did not alter maximum DO pressures (34). Several studies, however, showed that DO can improve post RP. In a study of 66 patients with PPI, Dubbelman et al., found a pre-operative DO rate of 26% which improved to 21% post RP (24). Giannantoni et al., found that 61% of patients had pre-existing DO. After 3 years of follow-up, the post RP DO rate was 56%, some of which were de novo (19). Similarly Matsukawa et al., found that DO disappeared in 54% of patients with pre-existing DO post RP, while 21% of patients developed de novo DO (23). Comparable results were found by Slova et al., who reported that storage symptoms were significantly improved after open RP (35). Thus, the natural progression of DO post RP can be variable. Some patients with pre-existing DO report an improvement while others stay the same, and some patients develop post RP de novo DO.
Implications of DO in men with SUI
Men with DO appear to have worse continence outcomes after a retroluminal transobturator (AdVance®) sling surgery (36-38). Conversely, the presence of DO does not seem to worsen the continence outcome post AUS surgery (27,28). However, de novo or persistent DO related symptoms occur commonly post AUS surgery and a patient needs to be counselled about this (39).
In general, we believe that it is important to treat DO first. This will have the effect of reducing the overall PPI and may make the component of SUI more apparent. Sometimes, the PPI may improve to the point where the patient may not need their SUI treated (14), or be treatable with a male sling rather than an AUS.
Management of post prostatectomy DO
The management of DO related PPI is determined by its severity and by the presence of ISD, DU and poor compliance (PC). Assessment should comprise of urinalysis, urine culture, 24-hour pad weight, total number of pads, post void residual volume (PVR) and UDS. The subjective impact of PPI may be assessed with a validated questionnaire such as the international consultation on incontinence (ICIQ)—overactive bladder questionnaire.
The three main treatment approaches are similar to non-prostate cancer patients, they are as follows:
- Behavioral therapy (bladder training, biofeedback, pelvic floor muscle therapy, and restricting fluid intake);
- Pharmacologic therapy (anticholinergic and β3 agonists);
- Surgical therapy [Intravesical onabotulinumtoxinA (Botox®), SNM, urinary diversion].
There is a relative deficiency of data reporting the use of anticholinergic medications in post RP patients. However Leach et al., demonstrated that anticholinergics significantly decreased pad score in patient with DO prior to AUS insertion (14). Mirabegron, the selective β3 agonist, has similar efficacy to anticholinergics but with less side effects, that may benefit patients with PPI DO. But there is no data reported of its use in the post RP population.
Surgical treatments include intravesical Botox®, SNM, and as a last resort, urinary diversion. Intravesical Botox® has an efficacy rate of 30–86% (40-42). However, Botox has a limited duration of benefit and repeat treatments are needed. There is also a significant risk of urinary retention (about 5%) and the patient may be required to perform clean intermittent self-catheterization (CISC) (43). Intravesical Botox may be an unattractive treatment if AUS is planned, as repeated cystoscopy or CISC may increase the risk of cuff erosion.
SNM is an alternative to intravesical Botox® on theoretical grounds. At this stage there are relatively few data about SNM in post RP population. For DO generally, SNM has a success rate of 53% to 80% (44-46). SNM does not cause retention and may treat the other forms of bladder dysfunction that can be found in association with DO, in particular DU, with success rates of 66.7% to 87.4% (47). Thus, SNM is potentially preferable to intravesical Botox® in treating post PR DO.
For severe refractory DO post RP, continued pad use or major open surgery may be the only options remaining. Augmentation cystoplasty is associated with high rates of CISC (75%) (48), and is not recommended, as this increases the risk of device urethral erosion. Creation of a urinary diversion remains another viable option, particularly in patients who might be deemed unsuitable for reconstructive bladder surgery (49). These treatments must be seen as a last resort.
Post prostatectomy DU
Incidence and diagnosis of post prostatectomy DU
The International Continence Society (ICS) defines DU as “a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or failure to achieve complete bladder emptying within a normal time span” (50). Some patients have pre-existing DU and others develop it de novo (7), mainly as a result of denervation injury during the RP. Interestingly Chung et al., postulated that minimally invasive surgery has a higher risk of causing DU as it involves a posterior approach to the dissection of the seminal vesicles where the pelvic nerves are situated. During an open RP, dissection is preformed closer to the seminal vesicles due to traction on the prostate, sparing nerves at the base of the bladder (51). In the community, the prevalence of DU is about 9% to 23% in men less than 50 years, increasing to about 48% in men older than 70 (52). Post RP DU appears to be common; Chung et al. reported that 41% of patient post RP had DU (51). Similarly, Porena et al., found DU in 29–61% of patients post RP, of which 47% are de novo (32).
Studies reporting the incidence of DU post RP have limitations. Firstly, there is no consensus on which urodynamic method should be used to diagnose DU. Described methods include the Bladder Contractility Index (BCI), the presence of abdominal straining during voiding and arbitrary urodynamic cutoffs such as PdetQmax <20 cmH2O or a Qmax <15 mL/sec. PdetQmax in men with ISD may be falsely low due to reduced urethral resistance and formulas based on this may be inaccurate to diagnose DU (53). Isometric detrusor contraction pressure (Piso) may be a more accurate method to diagnose DU. It is measured by gently occluding the penile urethra during voiding, with a Piso of less than 50 cmH2O being diagnostic of DU (53). Secondly, not all studies compared pre and post op urodynamics findings, and therefore the true effect of RP on detrusor contractility is not fully appreciated.
Implications of DU in men with SUI
Men with DU often void with abdominal straining due to insufficient detrusor strength. This can also be a learned behavior, as patients with decreased sphincteric resistance may find it easier and faster to void by straining (12). Therefore some concern exists in placing a male sling to treat SUI in men with DU, as the sling is designed to create a fixed resistance and may cause urinary retention. One study tried to alleviate some of these concerns. Han et al., examined 50 patients with DU vs. 42 patients with normal contractility who had sling procedures. They found no significant differences in post sling PVR and Patient Global Impression of Improvement (PGI-I). In addition, there were no differences in those who were valsalva voiders (Pabd >20 cmH2O during voiding) vs. normal voiders (54). The authors concluded that placing a male sling in these patients with DU was safe, but we certainly need more similar studies to confirm this.
Regarding the choice of male sling in DU patients, the retroluminal transobturator sling (AdVance sling®) offers an advantage over the more compressive quadratic sling (Virtue sling®) (55). The AdVance® sling acts by relocating the bulbar urethra and causes minimal compression, whereas the compressive quadratic Virtue sling® is typically tensioned to a pressure of 60 cmH2O. DU patients may not be able to generate this pressure and may not be able to void (56).
Placing an AUS in patients with DU or who are Valsalva voiders post RP appears to be safe and effective. The cuff is cycled open with relief of obstruction during voiding. Studies have demonstrated no increased risk of raised PVR or urinary retention post AUS in these patients (18,57). As DU can present with both voiding and storage symptoms, patients still have to be counselled post SUI surgery about the possibility of persistence of common DU symptoms including urgency, weak stream, straining to void, and nocturia (58).
Management of post prostatectomy DU
If a patient is able to empty well during voiding after SUI surgery, he can be advised to continue to do double voiding or use abdominal straining. If there is decreased bladder sensation, the patient may have to do timed voiding. In patients who have pre RP DU or in those with an acontractile bladder who are not able to empty the bladder even with abdominal straining, it is anticipated they may have to continue or start doing CISC even after SUI surgery. It is not advisable to perform CISC through a urethral AUS cuff due to the high risk of erosion. A bladder neck AUS cuff is also not recommended as it is considered technically difficult and risky in a post RP patient where the anastomotic area may be scarred.
In this setting, an adjustable transobturator male sling (ATOMS®) may be considered although there has been no published data about this. Unlike the AUS which circumferentially compresses the urethra, the ATOMS® only compresses the bulbar urethra dorsally, leaving the ventral and lateral blood supply intact. The bulbospongiosus muscle is also left intact and acts as an additional protective layer between the device and the urethra. Hoda et al., reported no case of urethral erosion in their series of 99 patients with ATOMS® with a mean follow-up of 17.8 months, although no patient needed to do CISC (59). In an abstract, Law et al. reported on one patient who needed to start CISC three times a day, in their series of eight patients who were implanted with the ATOMS®, and there was no device erosion in that patient (60).
SNM is an option for patients with DU. A meta-analysis by Gross et al., found that patients with DU had statistically significant increase in voided volume and a decrease in mean PVR after SNM treatment (61). In the non RP population the place of SNM in DU is well established. SNM is an effective treatment option for DU with excellent success rates. However, there are currently no published data on SNM in patients with post RP DU. Further research may demonstrate the place of SNM in patients with mixed PPI. If the DU can be successfully treated with SNM first, it may obviate the need for CISC, and simplify the subsequent treatment of the SUI.
Post prostatectomy impaired bladder compliance
Incidence of post prostatectomy impaired bladder compliance
Bladder compliance is calculated by dividing the volume change (ΔV) by the change in detrusor pressure (ΔPdet) during that change in volume (mL/cmH2O). The ICS recommends two standard points be used for determination of compliance, firstly the detrusor pressure at the start of bladder filling and secondly, the detrusor pressure at cystometric capacity or before the start of any detrusor contraction (51). Poor bladder compliance, is defined as significant increases in Pdet with small increments in bladder volume and may lead to incontinence and damage of the upper urinary tract (62,63). Various definitions and bladder pressure criterion have been advocated for poor compliance. Chou et al., recommend <10 mL/cmH2O (64). Weld et al., reported higher incidences of upper tract damage and vesicoureteral reflux in the spinal cord injury population with bladder compliance of <12.5 mL/cmH2O (65), while others suggest <20 mL/cmH2O as poor compliance (66).
Several studies have examined for impaired compliance in patients with PPI. Ficazzola et al., found that impaired bladder compliance was present in 5% (6). Conversely, Giannantoni et al., reported that 28.1% of patients demonstrated evidence of impaired bladder compliance which was defined as change in detrusor pressure of 20 mL/cmH2O at 3 years post RP (66). Gomha et al., noted approximately 24.6% of patients demonstrated impaired compliance, of which 9.8% had PC (defined as >10 mL/cmH2O) (18). While there seems to be variation in the reported incidence of impaired compliance after RP, it certainly is something that should be borne in mind by the clinician. It must be noted that impaired compliance in severely incontinent patients with ISD may be artefactual, due to supra-physiologic filling of chronically under filled bladders during urodynamics, and may be over reported.
Management of post prostatectomy impaired bladder compliance
Impaired or poor bladder compliance can be managed with observation and conservative measures such as timed voiding, anticholinergics, beta-3-agonists, or intravesical Botox® (28). Anticholinergics medications are effective in increasing bladder capacity, decreasing bladder filling pressure, and improving bladder compliance (67-69). In addition to improving compliance, Watanabe et al., demonstrated improved hydronephrosis and vesicoureteral reflux with anticholinergics (70). Similarly, mirabegron a β3-adrenoceptor agonist, improves cystometric capacity and bladder compliance, and it lowers vesicoureteral reflux grade in patients with the poorly compliant bladder and is an option for those who are intolerant of anticholinergics (71). Intravesical Botox has been indirectly used to treat PC in several studies examining the effect of Botox® on DO. In addition to increasing bladder capacity, Botox® has been shown to improve bladder compliance (70,72-74).
In the setting of PPI, when SUI surgery is considered, bladder compliance becomes an important consideration. Any procedure that obstructs the bladder outlet such as male sling or AUS could increase bladder pressure that may be transmitted to the upper tracts, potentially placing the kidneys at risk. Appropriate management of impaired compliance with the goal of reducing bladder pressures should be advocated prior to undertaking SUI surgery. Treatments should be evaluated with repeat urodynamics to assess for treatment success, and to ensure that SUI surgery can proceed without undue risk of upper urinary tract deterioration over the long-term.
Implications of impaired compliance in men with SUI
There is limited data in the literature about the safety of a male sling in terms of upper tract and renal function preservation in patients with poor bladder compliance. Logically, a tight compressive sling (quadratic Virtue sling®) may be contraindicated and a non-occlusive sling may be safer. Habashy et al. reported on 20 patients with PC who had the AdVance® sling, and PC is not predictive of worse continence outcome. However, they did not report on the post-sling incidence of renal failure or hydronephrosis (36).
It is still unknown if poor bladder compliance is an absolute contraindication to AUS surgery in the non-neurogenic, non-irradiated patient population. There appears to be a tendency towards worse continence results in those with impaired compliance on pre-operative urodynamic studies (28). Other studies, however, have failed to corroborate these findings (12,27). Preservation of continence status post AUS surgery may also be an ominous sign of potential upper tract damage (28). While no safe cutoff detrusor pressures for the implantation of AUS has been established, patients with mildly impaired bladder compliance may still undergo insertion of an AUS. In these patients, long-term follow-up of the upper tracts with periodic serum creatinine measurement, and renal ultrasound should be employed to screen for upper urinary tract deterioration (75). These patients may also be advised to do timed voiding in order to avoid reaching the threshold bladder volumes that result in high bladder pressures. There remains a subset of patients with persistently elevated detrusor pressures, or cannot be relied upon to do timed voiding, or have evidence of pre-existing renal impairment/hydronephrosis, who ultimately may not be suitable for SUI surgery. These patients should be counseled accordingly, as the PPI may be serving as a “pop-off” mechanism, protecting their upper tracts.
Conclusions
SUI remains the most common cause of PPI, but bladder dysfunction in the form of DU, DO and PC are important causes of PPI that must not be ignored. All can occur pre RP or can arise de-novo and can exist alone or in combination with SUI. Bladder dysfunction can affect the outcome of SUI surgery, thus each patient must be treated on an individual basis. Patients with SUI and DO are recommended to have their DO treated first. Patients with DU and SUI must be counselled that they may not be able to void after sling surgery. Patient with PC may need their compliance treated to prevent upper tract damage prior to SUI surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol 1998;160:2418-24. [Crossref] [PubMed]
- Kielb S, Dunn RL, Rashid MG, et al. Assessment of early continence recovery after radical prostatectomy: patient reported symptoms and impairment. J Urol 2001;166:958-61. [Crossref] [PubMed]
- Jønler M, Madsen FA, Rhodes PR, et al. A prospective study of quantification of urinary incontinence and quality of life in patients undergoing radical retropubic prostatectomy. Urology 1996;48:433-40. [Crossref] [PubMed]
- Kim JC, Cho KJ. Current trends in the management of post-prostatectomy incontinence. Korean J Urol 2012;53:511-8. [Crossref] [PubMed]
- Ficazzola MA, Nitti VW. The etiology of post-radical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J Urol 1998;160:1317-20. [Crossref] [PubMed]
- Chao R, Mayo ME. Incontinence after radical prostatectomy: detrusor or sphincter causes. J Urol 1995;154:16-8. [Crossref] [PubMed]
- Kleinhans B, Gerharz E, Melekos M, et al. Changes of urodynamic findings after radical retropubic prostatectomy. Eur Urol 1999;35:217-21; discussion 221-2. [Crossref] [PubMed]
- Hellström P, Lukkarinen O, Kontturi M. Urodynamics in radical retropubic prostatectomy. Scand J Urol Nephrol 1989;23:21-4. [Crossref] [PubMed]
- Presti JC Jr, Schmidt RA, Narayan PA, et al. Pathophysiology of urinary incontinence after radical prostatectomy. J Urol 1990;143:975-8. [Crossref] [PubMed]
- Foote J, Yun S, Leach GE. Postprostatectomy incontinence. Pathophysiology, evaluation, and management. Urol Clin North Am 1991;18:229-41. [PubMed]
- Groutz A, Blaivas JG, Chaikin DC, et al. The pathophysiology of post-radical prostatectomy incontinence: a clinical and video urodynamic study. J Urol 2000;163:1767-70. [Crossref] [PubMed]
- Goluboff ET, Chang DT, Olsson CA, et al. Urodynamics and the etiology of post-prostatectomy urinary incontinence: the initial Columbia experience. J Urol 1995;153:1034-7. [Crossref] [PubMed]
- Leach GE, Trockman B, Wong A, et al. Post-prostatectomy incontinence: urodynamic findings and treatment outcomes. J Urol 1996;155:1256-9. [Crossref] [PubMed]
- Desautel MG, Kapoor R, Badlani GH. Sphincteric incontinence: the primary cause of post-prostatectomy incontinence in patients with prostate cancer. Neurourol Urodyn 1997;16:153-60. [Crossref] [PubMed]
- Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J Urol 1997;157:233-6. [Crossref] [PubMed]
- Winters JC, Appell RA, Rackley RR. Urodynamic findings in postprostatectomy incontinence. Neurourol Urodyn 1998;17:493-8. [Crossref] [PubMed]
- Gomha MA, Boone TB. Voiding patterns in patients with post-prostatectomy incontinence: urodynamic and demographic analysis. J Urol 2003;169:1766-9. [Crossref] [PubMed]
- Giannantoni A, Mearini E, Di Stasi SM, et al. Assessment of bladder and urethral sphincter function before and after radical retropubic prostatectomy. J Urol 2004;171:1563-6. [Crossref] [PubMed]
- Huckabay C, Twiss C, Berger A, et al. A urodynamics protocol to optimally assess men with post-prostatectomy incontinence. Neurourol Urodyn 2005;24:622-6. [Crossref] [PubMed]
- Kielb SJ, Clemens JQ. Comprehensive urodynamics evaluation of 146 men with incontinence after radical prostatectomy. Urology 2005;66:392-6. [Crossref] [PubMed]
- Ventimiglia B, Tsirgiotis A, Fanzone I, et al. Urologia 2011;78:82-5. [Urinary incontinence after radical prostatectomy. Neurophysiological and urodynamic diagnosis]. [Crossref] [PubMed]
- Matsukawa Y, Hattori R, Komatsu T, et al. De novo detrusor underactivity after laparoscopic radical prostatectomy. Int J Urol 2010;17:643-8. [Crossref] [PubMed]
- Dubbelman Y, Groen J, Wildhagen M, et al. Quantification of changes in detrusor function and pressure-flow parameters after radical prostatectomy: relation to postoperative continence status and the impact of intensity of pelvic floor muscle exercises. Neurourol Urodyn 2012;31:637-41. [Crossref] [PubMed]
- Majoros A, Bach D, Keszthelyi A, et al. Urinary incontinence and voiding dysfunction after radical retropubic prostatectomy (prospective urodynamic study). Neurourol Urodyn 2006;25:2-7. [Crossref] [PubMed]
- Pérez LM, Webster GD. Successful outcome of artificial urinary sphincters in men with post-prostatectomy urinary incontinence despite adverse implantation features. J Urol 1992;148:1166-70. [Crossref] [PubMed]
- Thiel DD, Young PR, Broderick GA, et al. Do clinical or urodynamic parameters predict artificial urinary sphincter outcome in post-radical prostatectomy incontinence? Urology 2007;69:315-9. [Crossref] [PubMed]
- Trigo Rocha F, Gomes CM, Mitre AI, et al. A prospective study evaluating the efficacy of the artificial sphincter AMS 800 for the treatment of postradical prostatectomy urinary incontinence and the correlation between preoperative urodynamic and surgical outcomes. Urology 2008;71:85-9. [Crossref] [PubMed]
- Afraa TA, Campeau L, Mahfouz W, et al. Urodynamic parameters evolution after artificial urinary sphincter implantation for post-radical prostatectomy incontinence with concomitant bladder dysfunction. Can J Urol 2011;18:5695-8. [PubMed]
- Lai HH, Hsu EI, Boone TB. Urodynamic testing in evaluation of postradical prostatectomy incontinence before artificial urinary sphincter implantation. Urology 2009;73:1264-9. [Crossref] [PubMed]
- Steiner MS. Continence-preserving anatomic radical retropubic prostatectomy. Urology 2000;55:427-35. [Crossref] [PubMed]
- Porena M, Mearini E, Mearini L, et al. Voiding dysfunction after radical retropubic prostatectomy: more than external urethral sphincter deficiency. Eur Urol 2007;52:38-45. [Crossref] [PubMed]
- Ventimiglia B, Sigona M, Di Dio A, et al. Urinary incontinence and neuropathy after radical prostatectomy: diagnosis and treatment. Urologia 2015;82:42-5. [Crossref] [PubMed]
- Constantinou CE, Freiha FS. Impact of radical prostatectomy on the characteristics of bladder and urethra. J Urol 1992;148:1215-9; discussion 1219-20. [Crossref] [PubMed]
- Slova D, Lepor H. The short-term and long-term effects of radical prostatectomy on lower urinary tract symptoms. J Urol 2007;178:2397-400; discussion 2400-1. [Crossref] [PubMed]
- Habashy D, Losco G, Tse V, et al. Mid-term outcomes of a male retro-urethral, transobturator synthetic sling for treatment of post-prostatectomy incontinence: Impact of radiotherapy and storage dysfunction. Neurourol Urodyn 2017;36:1147-50. [Crossref] [PubMed]
- Zuckerman JM, Edwards B, Henderson K, et al. Extended outcomes in the treatment of male stress urinary incontinence with a transobturator sling. Urology 2014;83:939-45. [Crossref] [PubMed]
- Crites MA, Sorial A, Ghoniem GM. Risk factors for male slings: a comparative study of two techniques. Urology 2011;78:192-6. [Crossref] [PubMed]
- Lai HH, Boone TB. Implantation of artificial urinary sphincter in patients with post-prostatectomy incontinence, and preoperative overactive bladder and mixed symptoms. J Urol 2011;185:2254-9. [Crossref] [PubMed]
- Rovner E, Kennelly M, Schulte-Baukloh H, et al. Urodynamic results and clinical outcomes with intradetrusor injections of onabotulinumtoxinA in a randomized, placebo-controlled dose-finding study in idiopathic overactive bladder. Neurourol Urodyn 2011;30:556-62. [Crossref] [PubMed]
- Denys P, Le Normand L, Ghout I, et al. Efficacy and safety of low doses of onabotulinumtoxinA for the treatment of refractory idiopathic overactive bladder: a multicentre, double-blind, randomised, placebo-controlled dose-ranging study. Eur Urol 2012;61:520-9. [Crossref] [PubMed]
- Schmid DM, Sauermann P, Werner M, et al. Experience with 100 cases treated with botulinum-A toxin injections in the detrusor muscle for idiopathic overactive bladder syndrome refractory to anticholinergics. J Urol 2006;176:177-85. [Crossref] [PubMed]
- Karsenty G, Denys P, Amarenco G, et al. Botulinum toxin A (Botox) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic literature review. Eur Urol 2008;53:275-87. [Crossref] [PubMed]
- Davis T, Makovey I, Guralnick ML, et al. Sacral neuromodulation outcomes for the treatment of refractory idiopathic detrusor overactivity stratified by indication: Lack of anticholinergic efficacy versus intolerability. Can Urol Assoc J 2013;7:176-8. [Crossref] [PubMed]
- Scheepens WA, van Koeveringe GA, de Bie RA, et al. Urodynamic results of sacral neuromodulation correlate with subjective improvement in patients with an overactive bladder. Eur Urol 2003;43:282-7. [Crossref] [PubMed]
- Van Voskuilen AC, Oerlemans DJ, Weil EH, et al. Medium-term experience of sacral neuromodulation by tined lead implantation. BJU Int 2007;99:107-10. [Crossref] [PubMed]
- Saber-Khalaf M, Abtahi B, Gonzales G, et al. Sacral neuromodulation outcomes in male patients with chronic urinary retention. Neuromodulation 2015;18:329-34; discussion 334. [Crossref] [PubMed]
- Hasan ST, Marshall C, Robson WA, et al. Clinical outcome and quality of life following enterocystoplasty for idiopathic detrusor instability and neurogenic bladder dysfunction. Br J Urol 1995;76:551-7. [Crossref] [PubMed]
- Singh G, Wilkinson JM, Thomas DG. Supravesical diversion for incontinence: a long-term follow-up. Br J Urol 1997;79:348-53. [Crossref] [PubMed]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116-26. [Crossref] [PubMed]
- Chung DE, Dillon B, Kurta J, et al. Detrusor underactivity is prevalent after radical prostatectomy: A urodynamic study including risk factors. Can Urol Assoc J 2013;7:E33-7. [Crossref] [PubMed]
- Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol 2014;65:389-98. [Crossref] [PubMed]
- Jura YH, Comiter CV. Urodynamics for postprostatectomy incontinence: when are they helpful and how do we use them? Urol Clin North Am 2014;41:419-27. viii. [Crossref] [PubMed]
- Han JS, Brucker BM, Demirtas A, et al. Treatment of post-prostatectomy incontinence with male slings in patients with impaired detrusor contractility on urodynamics and/or who perform Valsalva voiding. J Urol 2011;186:1370-5. [Crossref] [PubMed]
- Rehder P, Mitterberger MJ, Pichler R, et al. The 1 year outcome of the transobturator retroluminal repositioning sling in the treatment of male stress urinary incontinence. BJU Int 2010;106:1668-72. [Crossref] [PubMed]
- Comiter CV, Dobberfuhl AD. The artificial urinary sphincter and male sling for postprostatectomy incontinence: Which patient should get which procedure? Investig Clin Urol 2016;57:3-13. [Crossref] [PubMed]
- Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. [Crossref] [PubMed]
- Hoag N, Gani J. Underactive Bladder: Clinical Features, Urodynamic Parameters, and Treatment. Int Neurourol J 2015;19:185-9. [Crossref] [PubMed]
- Hoda MR, Primus G, Fischereder K, et al. Early results of a European multicentre experience with a new self-anchoring adjustable transobturator system for treatment of stress urinary incontinence in men. BJU Int 2013;111:296-303. [Crossref] [PubMed]
- Law MC, Chan SY, Cheung HY, et al. Adjustable transobturator male system (ATOMS) for male postprostatecomy strees urinary incontinence: iniital multi-centre experience in Hong Kong. ICS 2012:413.
- Gross C, Habli M, Lindsell C, et al. Sacral neuromodulation for nonobstructive urinary retention: a meta-analysis. Female Pelvic Med Reconstr Surg 2010;16:249-53. [Crossref] [PubMed]
- Wyndaele JJ, Gammie A, Bruschini H, et al. Bladder compliance what does it represent: can we measure it, and is it clinically relevant? Neurourol Urodyn 2011;30:714-22. [Crossref] [PubMed]
- McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 1981;126:205-9. [Crossref] [PubMed]
- Chou FH, Ho CH, Chir MB, et al. Normal ranges of variability for urodynamic studies of neurogenic bladders in spinal cord injury. J Spinal Cord Med 2006;29:26-31. [Crossref] [PubMed]
- Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol 2000;163:1228-33. [Crossref] [PubMed]
- Giannantoni A, Mearini E, Zucchi A, et al. Bladder and urethral sphincter function after radical retropubic prostatectomy: a prospective long-term study. Eur Urol 2008;54:657-64. [Crossref] [PubMed]
- Stöhrer M, Murtz G, Kramer G, et al. Propiverine compared to oxybutynin in neurogenic detrusor overactivity--results of a randomized, double-blind, multicenter clinical study. Eur Urol 2007;51:235-42. [Crossref] [PubMed]
- Cameron AP, Clemens JQ, Latini JM, et al. Combination drug therapy improves compliance of the neurogenic bladder. J Urol 2009;182:1062-7. [Crossref] [PubMed]
- Christoph F, Moschkowitsch A, Kempkensteffen C, et al. Long-term efficacy of tolterodine and patient compliance in pediatric patients with neurogenic detrusor overactivity. Urol Int 2007;79:55-9. [Crossref] [PubMed]
- Watanabe M, Yamanishi T, Honda M, et al. Efficacy of extended-release tolterodine for the treatment of neurogenic detrusor overactivity and/or low-compliance bladder. Int J Urol 2010;17:931-6. [Crossref] [PubMed]
- Kamei J, Furuta A, Akiyama Y, et al. Video-urodynamic effects of mirabegron, a beta3 -adrenoceptor agonist, in patients with low-compliance bladder. Int J Urol 2015;22:956-61. [Crossref] [PubMed]
- Karsenty G, Reitz A, Lindemann G, et al. Persistence of therapeutic effect after repeated injections of botulinum toxin type A to treat incontinence due to neurogenic detrusor overactivity. Urology 2006;68:1193-7. [Crossref] [PubMed]
- Klaphajone J, Kitisomprayoonkul W, Sriplakit S. Botulinum toxin type A injections for treating neurogenic detrusor overactivity combined with low-compliance bladder in patients with spinal cord lesions. Arch Phys Med Rehabil 2005;86:2114-8. [Crossref] [PubMed]
- Schurch B, Stohrer M, Kramer G, et al. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000;164:692-7. [Crossref] [PubMed]
- Hussain M, Greenwell TJ, Venn SN, et al. The current role of the artificial urinary sphincter for the treatment of urinary incontinence. J Urol 2005;174:418-24. [Crossref] [PubMed]


