How and why to take a Martius labial interposition flap in female urology
Introduction
The Martius modified labial fat pad flap (MMLFPF, referred to commonly as the Martius flap) remains the simplest and most versatile interposition tissue for complex vaginal surgery. In 1928 Martius described a labial flap of bulbocavernosus muscle for urethrovaginal fistula repair (1), which was later modified multiple times, and in modern usage generally refers to a labium majora fat pad flap without muscle (2).
For urological indications following vaginal procedures where tissue integrity is a concern, it is placed between the periurethral or perivesical fascia and anterior vaginal wall closure. Whether operating in large academic groups or those for whom such cases arise only sporadically in their practice, all surgeons who wish to maximize success at first procedure will find it an extremely useful tool. Here we describe flap harvest and placement and the justification for its use.
Technique
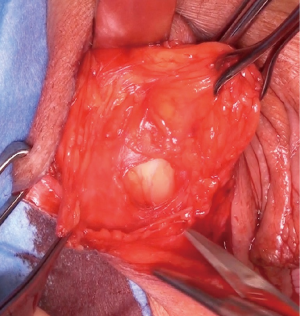
After completion of the main vaginal procedure a swab is placed in the vagina and any vaginal retractor used is either removed or manipulated to permit easy access to the labia majora.
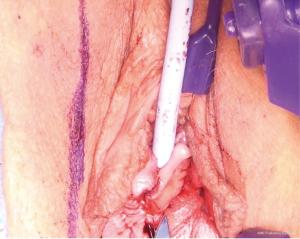
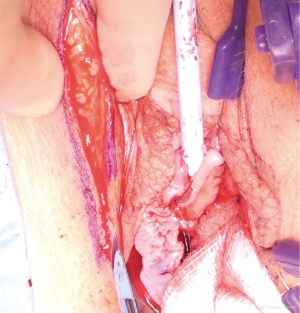
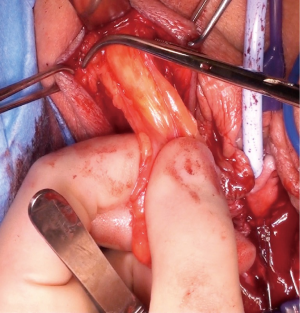
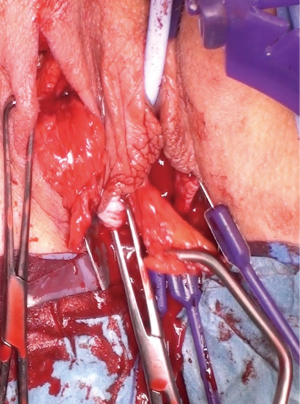
The most dependent edge of the labia majora is marked and incised from level with the mons pubis posteriorly (Figure 1), extending if more length is required, to expose the bright yellow fibrofatty pad (Figure 2). There is a natural tissue plane around the pad where medial (Figure 3) and lateral (Figure 4) dissection can be facilitated by spreading with curved scissors and diathermy, ensuring that posteriorly the lateral attachment through which the blood supply from a branch of the internal pudendal is maintained.
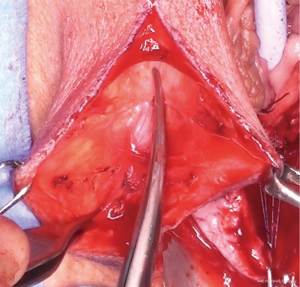
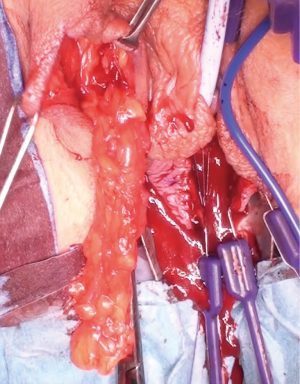
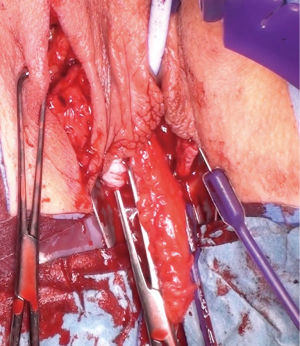
Posterior dissection is then performed (Figure 5) and the fat pad freed for the length required to transpose into the vagina and cover the area of surgery in a tension free manner, anything from 8–15 cm in general. Care should be taken to stay lateral to the bulbocavernosus and ischiocavernosus muscles. Too medial a dissection includes these structures and results in significant bleeding. Too superficial a dissection risks scar deformity.
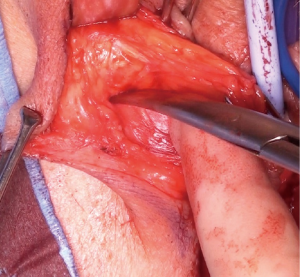
Superiorly the external pudendal artery and inferiorly the internal pudendal artery supply are reasonably predictable and form a plexus within the flap. Once the desired length is achieved the flap is divided, most commonly at its superior margin (Figure 6) with a Roberts or right-angled clamp for pedicle control, followed by division and suture ligation with 3/0 or 2/0-vicryl, (Figure 7), leaving a broad vascular inferior base to the flap that is practical for most purposes.
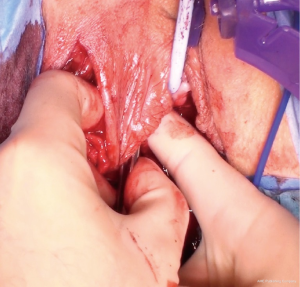
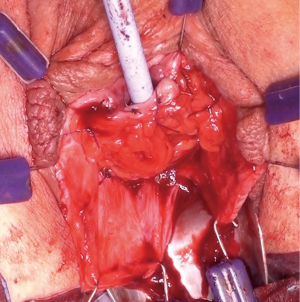
A tunnel connecting the pedicle base to the vaginal wound is formed by blunt dissection with fingers and Roberts clamp and then widened to admit at least two fingers (to alleviate compression on pedicle blood supply) (Figure 8). the flap is transferred gently through using a Satinsky clamp, which is the perfect configuration for this manoeuvre (Figures 9,10). The flap is positioned over the operative field requiring reinforcement and sutured to the peri-vaginal fascia without tension with 2-0 or 3-0 interrupted absorbable sutures to prevent migration (Figure 11). Peri-urethral tissue may be used to suture the flap but is weaker and more liable to tear.
Excess flap length should be trimmed as the benefit of the flap does not depend on a having a large mass of tissue. If additional tissue is required to support a large surgical field, bilateral flaps can be used. In the extremely uncommon event of insufficient vaginal skin or severe stenosis the flap can be pre-planned to include a full-thickness island of hairless skin from the medial labia majora surface (3).
The vaginal wound is closed as usual, and labial wound closed in layers with absorbable suture over a small suction drain (such as a Minivac) and a pressure dressing is applied (such as Opsite spray to skin, gauze and foam tape on tension) (Figure 12). It is important to cover the fat pad completely during closure—an exposed fat pad tends to be associated with a dragging sensation radiating from the labia to the vagina. The drain and pressure dressing are removed at 24–48 hours and other post-operative care determined by the primary procedure.
Uses
Tissue interposition can alleviate the surgical dilemmas of overlapping suture lines and poor quality tissue, create a layered closure in tissue where there may not be layers to close, and encourage neovascularity thereby improving healing and cure rates.
Labial fat makes an excellent interposition tissue for the following reasons:
- The fibrous component makes it a strong flap despite its adiposity;
- It has 2 pedicles and will survive on either, giving positional flexibility for multiple vaginal applications and defect locations;
- It is conveniently located in the same surgical field as vaginal surgery, unlike alternative flaps such as peritoneum, gracilis, or omentum;
- It is not bulky and can be tailored in-situ without compromise;
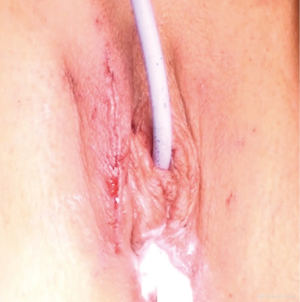
- It is surprisingly cosmetic as the superficial labial fat is not disturbed and the labial incision quickly becomes inconspicuous (Figure 13);
The main urological indications for the MMLFPF are
- To improve healing and reduce fistulae or recurrence in urethral diverticulectomy (4,5), urethral mesh excision (4,6), vaginal repair of urethrovaginal (6) and vesicovaginal fistulae (6-8), and bladder neck closure for urethral erosion (9);
- To prevent recurrent scarring of the urethra following urethrolysis or urethral stricture repair (7,10,11);
- Protection of the fragile urethra and vagina from stress incontinence surgery complications (12); tensioning a sling may however be more difficult to gauge over a Martius flap.
Operative scenarios particularly deserving of interposition are fibrotic, scarred or previously irradiated tissue, tenuous periurethral fascia and revision surgery (13). Especially complex are those with failed prior diverticulectomy, mesh excision or fistula repair attempts (13). Reoperation for failure or complications can be tremendously debilitating with long-term adverse sequelae and medicolegal implications (14).
Potential uses of the Martius flap are not limited to those described. Extended applications that have been more recently reported include:
- Salvage of vaginally eroded synthetic mesh (midurethral sling or pelvic organ prolapse kit) by covering with a Martius flap (preserving tape function in preference to excising the eroded segment) (15,16);
- Neovagina formation using a further alliteration of the Martius flap (17);
- Transvaginal repair of the particularly challenging neobladder-vaginal fistula after radical cystectomy and orthotopic diversion (18,19);
- Paediatric vaginal reconstruction (after pretreatment with topical oestrogen)(20).
Outcomes
The relative rarity of female urethral reconstruction in developed countries (for example the small numbers of cases per year in even the quaternary referral centres publishing results) means an absence of large case–control series or trials.
Worldwide, vesicovaginal fistula repair would seem by numbers to be the most commonly occurring scenario where a MMLFPF could be of assistance, birth trauma being the commonest cause, with estimates of between one to two million women worldwide suffering from obstetric fistula (13,21,22). Another is reconstruction after vaginal trauma resulting from a particularly horrific rise in sexual torture in war ravaged areas like the Congo (23,24). The question has been raised regarding the necessity of using interposition tissue in obstetric vaginal fistulae and there is a trend in this indication to limit flap use to only the most complex cases (25). Browning’s series of 440 obstetric VVF repairs (26) proposed that its use in this scenario be abandoned because of complications and lack of benefit (7) with a higher rate of postoperative incontinence in those with Martius flap interposition (44.9% vs. 16.5%). As discussed previously (4) the poorer continence outcomes may have been confounded by indication as stratified analyses suggest those fistulae repaired with a Martius flap may have been more complex (more difficult to repair and have known higher rates of incontinence). A second series of 81 genitourinary fistulae operated on by a single surgeon (27) of which 28 (34.6%) received a MMLFPF, found the addition of a flap made no difference in the overall closure rate (85.7% vs. 79.2%, P=0.347) nor the closure of fistula with continence (60.7% vs. 67.9%, P=0.260). This small unrandomized series suggests in women where the fistula characteristics are thought to be poor enough to warrant interposition tissue, that its use yields surgical outcomes equivalent to those in milder cases not needing interposition. By way of western case series contrast, DeLancey and McGuire series of 37 repairs of complex fistulae using MMLFPF in 35 patients yielded an overall cure of 86.5% (8).
The MMLFPF harvest carries little morbidity, takes little additional surgical time, improves healing, definitely does not worsen and does improve surgical outcomes in difficult cases. Good technique minimizes flap bulk and retraction from excessive tension, pedicle dissection or an inadequate tunnel. Rare reported complications include vaginal prominence of a bulky flap presenting as a vaginal mass (28). In terms of complications, Kasyan et al. (29) reported the highest rate (harvest site bleeding 19%, haematoma 5%, labial incision lymphorrhoea 13.5%, wound infection 5.4%) in their series of 37 patients. Long term patient reported outcomes were based on the 24 women contactable for follow up, of whom 4 reported cosmetic problems and two reported intermittent mild pain. Lee et al.’s (30) more recent prospective series of 122 patients undergoing MMLFPF surgery with a mean follow up of 85 months (6-202 months) recorded no perioperative complications, and low rates of long term complications of pain in 5%, numbness in 14% and labial distortion in 7%, which we feel is a more representative of general outcomes in experienced hands. Among sexually active patients, equivocal sexual function outcomes were recorded across different surgical indications, although it is difficult to control for other factors such as primary vaginal surgery indication, age, health status when assessing sexual function outcomes. Our previously reported series (4) found minimal morbidity with only 2 haematomas, 1 labial wound infection and very good cosmesis—with 79% of patients rating the final cosmetic appearance as good or excellent and only 1 (0.6%) rating it as unsatisfactory.
Stress incontinence resulting from the vaginal surgery or pre-existing urethral incompetence may persist, and although a Martius flap may provide some minor degree of urethral support and facilitate reestablishment of continence (25), total reliance on it as a sling can lead to disappointment (13,25). Stress incontinence can be addressed concomitantly or preferably at a later surgery, depending on the patient scenario (25).
Petrou’s small series of 8 was the first to examine patient self-perception at the Martius harvest site (31). Although 62% reported numbness at the harvest site and 3 reported lingering pain a year after surgery, only 1 reported interference with sexual relations and it was not associated with perceived cosmetic disfigurement. Elkins (32) reported a 25% incidence of dyspareunia over the Martius flap harvest site in the vagina in 35 women following vesicovaginal and rectovaginal fistula repair. Female sexual dysfunction following vaginal surgery in general is underreported. The primary pathology or vaginal procedure are most likely the cause of the sexual dysfunction (33) and it is feasible that the addition of the Martius flap might help with alleviating adverse effects on sexual function. The potential for Martius flap associated side effects such as asymmetry, wound pain and dyspareunia, although extremely low, should be a point made in preoperative counselling. Body image distress sufficient to warrant further surgery is extremely rare; injectable bulking agents could be used in rare cases of significant labial asymmetry (34). Regarding labial sensation, a technique described by Deng et al of insitu flap harvest approaching from the vaginal wound without a separate labial incision may allow preservation of sensation although it will limit the amount of tissue that can be harvested (35).
Conclusions
For surgeons operating in developed countries, the Martius modified labial fat pad flap is a valuable tool in vaginal reconstruction with increasingly novel applications. The rate of complications and cosmetic dissatisfaction is low and outweighs the trouble of a failed operation. Randomised studies are welcomed in areas where flap use is declining.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Martius H. Die operative Wiederhellstellung der volkommen fehlenden Harnhohare unde des Sclessmuskels derselben. Zentralbl Gynakol 1928;52:480-6.
- Sajjadi SG, Hortváth ÖP, Kalmár K. Martius flap: historical and anatomical considerations. Eur J Plast Surg 2012;35:711-6. [Crossref]
- Carr LK, Webster GD. Full-thickness cutaneous martius flaps: a useful technique in female reconstructive urology. Urology 1996;48:461-3. [Crossref] [PubMed]
- Malde S, Spilotros M, Wilson A, et al. The uses and outcomes of the Martius fat pad in female urology. World J Urol 2017;35:473-8. [Crossref] [PubMed]
- Nickles SW, Ikwuezunma G, MacLachlan L, et al. Simple vs complex urethral diverticulum: presentation and outcomes. Urology 2014;84:1516-9. [Crossref] [PubMed]
- Blaivas JG, Mekel G. Management of urinary fistulas due to midurethral sling surgery. J Urol 2014;192:1137-42. [Crossref] [PubMed]
- Lee D, Dillon BE, Zimmern PE. Long-term morbidity of Martius labial fat pad graft in vaginal reconstruction surgery. Urology 2013;82:1261-6. [Crossref] [PubMed]
- Elkins TE, DeLancey JO, McGuire EJ. The use of modified Martius graft as an adjunctive technique in vesicovaginal and rectovaginal fistula repair. Obstet Gynecol 1990;75:727-33. [PubMed]
- Willis H, Safiano NA, Lloyd LK. Comparison of transvaginal and retropubic bladder neck closure with suprapubic catheter in women. J Urol 2015;193:196-202. [Crossref] [PubMed]
- Blaivas JG, Santos JA, Tsui JF, et al. Management of urethral stricture in women. J Urol 2012;188:1778-82. [Crossref] [PubMed]
- Carey JM, Chon JK, Leach GE. Urethrolysis with Martius labial fat pad graft for iatrogenic bladder outlet obstruction. Urology 2003;61:21-5. [Crossref] [PubMed]
- Matte A, Delorme E. The Martius flap in stress urinary incontinence treated by suburethral sling. Prog Urol 2012;22:725-30. [Crossref] [PubMed]
- Abrams P, De Ridder D, De Vries C, et al. Obstetric fistula in the developing world. An International Consultation on Vesicovaginal Fistula. Marrakech, Morocco 2010 Oct 13.
- Hansen BL, Dunn GE, Norton P, et al. Long-term follow-up of treatment for synthetic mesh complications. Female Pelvic Med Reconstr Surg 2014;20:126-30. [Crossref] [PubMed]
- Mortimer A, Khunda A, Ballard P. Martius graft for TOT extrusion: a case series. Int Urogynecol J 2016;27:113-6. [Crossref] [PubMed]
- Al-Badr A. Martius graft for management of exposed vaginal prolapse mesh. J Minim Invasive Gynecol 2013;20:227-9. [Crossref] [PubMed]
- Green AE, Escobar PF, Neubaurer N, et al. The martius flap neovagina revisited. Int J Gynecol Cancer 2005;15:964-6. [Crossref] [PubMed]
- Carmel ME, Goldman HB, Moore CK, et al. Transvaginal neobladder vaginal fistula repair after radical cystectomy with orthotopic urinary diversion in women. Neurourol Urodyn 2016;35:90-4. [Crossref] [PubMed]
- Tunuguntla HS, Manoharan M, Gousse AE. Management of neobladder-vaginal fistula and stress incontinence following radical cystectomy in women: a review. World J Urol 2005;23:231-5. [Crossref] [PubMed]
- Baskin D, Tatlidede S, Karşidağ SH. Martius repair in urethrovaginal defects. J Pediatr Surg 2005;40:1489-91. [Crossref] [PubMed]
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the prevalence of obstetric fistula: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2013 30;13:246.
- De Ridder D. An update on surgery for vesicovaginal and urethrovaginal fistulae. Curr Opin Urol 2011;21:297-300. [Crossref] [PubMed]
- Dossa NI, Zunzunegui MV, Hatem M, et al. Fistula and other adverse reproductive health outcomes among women victims of conflict-related sexual violence: a population-based cross-sectional study. Birth 2014;41:5-13. [Crossref] [PubMed]
- Baelani I, Dünser MW. Facing medical care problems of victims of sexual violence in Goma/Eastern Democratic Republic of the Congo. Confl Health 2011;5:2. [Crossref] [PubMed]
- Ruminjo JK, Frajzyngier V, Bashir Abdullahi M, et al. Clinical procedures and practices used in the perioperative treatment of female genital fistula during a prospective cohort study. BMC Pregnancy Childbirth 2014;14:220. [Crossref] [PubMed]
- Browning A. Lack of value of the Martius fibrofatty graft in obstetric fistula repair. International Journal of Gynaecology & Obstetrics 2006;93:33-7. [Crossref] [PubMed]
- Tebeu PM, Fokom-Domgue J, Kengne Fosso G, et al. Comparative study of the outcome of surgical management of vesico-vaginal fistulas with and without interposition of the Martius graft: A Cameroonian experience. Prog Urol 2015;25:1225-31. [Crossref] [PubMed]
- Kobashi KC, Mee SL, Leach GE. Vaginal Herniation of a Martius Fat Pad Graft Presenting as a Vaginal Mass. Infect Urol. 2000;13.
- Kasyan G, Tupikina N, Pushkar D. Use of Martius flap in the complex female urethral surgery. Cent European J Urol 2014;67:202-7. [Crossref] [PubMed]
- Lee, Dillon BE, Zimmern PE. Long-term morbidity of Martius labial fat pad graft in vaginal reconstruction surgery. Urology 2013;82:1261-6. [Crossref] [PubMed]
- Petrou SP, Joyce J, Parra RO. Martius flap harvest site: patient self-perception. J Urol 2002;167:2098-9. [Crossref] [PubMed]
- Elkins TE, DeLancey JO, McGuire EJ. The use of modified Martius graft as an adjunctive technique in vesicovaginal and rectovaginal fistula repair. Obstet Gynecol 1990;75:727-733. [PubMed]
- Tunuguntla HS, Gousse AE. Female sexual dysfunction following vaginal surgery: a review. J Urol 2006;175:439-46. [Crossref] [PubMed]
- Fasola E, Gazzola R. Labia Majora Augmentation with Hyaluronic Acid Filler: Technique and Results. Aesthet Surg J 2016;36:1155-63. [Crossref] [PubMed]
- Deng D, Rutman M, Rodriguez L, et al. The in situ Martius flap, description of a new technique. Non-discussion Poster 330, International Continence Society, Montreal, 2005.