MRI-fusion biopsy: the contemporary experience
Introduction
Prostate magnetic resonance imaging (MRI) is becoming increasingly used in clinical practice in the diagnostic pathway for prostate cancer (1,2). MRI may add value as both a pre-biopsy risk assessment tool that may influence the decision whether to perform biopsy as well as a minimally invasive method for tumor localization to direct targeted biopsy (2). Incorporating MRI with MRI-US-fusion targeted biopsy has shown improved sensitivity for detecting high grade prostate cancer while reducing the detection of clinically insignificant disease (3,4). In this review, we present our contemporary experience with prostate MRI and MRI-US-fusion targeted biopsy, highlighting our institutional program development and outcomes.
Methods of MRI-targeted biopsy
Currently three techniques of MRI guidance are available for targeted prostate biopsy: visual estimation TRUS-guided biopsy (also referred to as cognitive fusion), in-bore MRI guided biopsy, and software based co-registration targeted biopsy with MRI to ultrasound fusion. Each method possesses its own advantages and disadvantages but to date, no prospective comparison of all three methods has been made.
Visual estimation TRUS-guided biopsy, in which the ultrasound operator aims the biopsy needle at the prostate area where the previously reviewed MRI demonstrates a lesion, allows rapid adaption of MRI-targeted biopsy into clinical practice and requires no additional equipment beyond the MRI and a conventional transrectal ultrasound. The technique does carry a learning curve in that there is no real-time feedback regarding needle placement accuracy and the biopsy is prone to human error without actual image overlay. The effectiveness of visual estimation targeted biopsy in detecting prostate cancer varies between studies, likely due to investigator experience and variable practices in imaging approach.
In bore MRI-guided biopsy is performed within the MRI gantry, by a radiologist, who plans the biopsy based upon an acquired MRI and confirms biopsy needle localization under repetitive MRI sequences. Typically, only a few targeted cores are taken and systematic sampling is not readily performed, leaving normal appearing prostate tissue unsampled. The advantages of this method are fewer sampled cores, visual feedback regarding the accuracy of needle placement, and, in theory, the reduced detection of insignificant tumors.
Software co-registered MRI targeted TRUS biopsy allows the operator to image the prostate using ultrasound, while a previously performed and annotated MRI is fused with the real-time ultrasound using a digital overlay, creating a three-dimensional reconstruction of the prostate, on which the previously marked regions of interest are identified. Spatial tracking of the ultrasound probe through mechanical or electromagnetic means allows accurate placement of the needle guide relative to the three-dimensional reconstruction. MRI-US-fusion targeted biopsy potentially has greater reproducibility due to less operator dependence by providing real time feedback of actual biopsied locations. The disadvantages include the cost of an additional device and the requirement for specialized operator training.
Considerations in selection of method
Practice logistics
Visual estimation TRUS-guided biopsy can be adopted into clinical practice without significant resources or change in the prostate biopsy pathway. In-bore MRI-guided biopsy, however, requires a longer procedure time, has a high cost, and is resource-intensive in that it requires prolonged access to a MRI scanner. Software co-registered MRI targeted TRUS biopsy can be instituted with limited changes in the typical TRUS-guided biopsy pathway. Prior to biopsy, the segmentation of the MRI images must be performed, including contouring the edge of the prostate and of the focal targets within the prostate. The software co-registration step also adds time to the biopsy procedure.
Who does the biopsy
All of the methods of targeted biopsy may be performed by either a urologist or radiologist. However, both visual estimation and software co-registration MRI targeted biopsy can be performed in the office and are most commonly performed by urologists. In-bore MRI targeted biopsy must be performed at the site of the MRI scanner and is most often performed by radiologists.
Cost
All three targeted biopsy methods bear the cost of a pre-biopsy MRI. Outside the economic burden of an MRI, visual estimation targeted biopsy does not require additional costs or resources. In-bore targeted biopsy has a substantial cost in that it requires prolonged access to the MRI scanner and as a result, limits the MRI use for other activities. Software co-registered MRI-US-fusion targeted biopsy requires the purchase of a MRI-US fusion platform.
Merits of fusion biopsy and our decision to use fusion
PROFUS study
Pre-biopsy prostate MRI along with targeted biopsy has the potential to correct the limitations of systematic biopsy including the ability to target patients with MRI detected abnormalities, obtain fewer false negatives which results in fewer repeat biopsies, achieve more accurate cancer classification, greater cancer core length, better grade concordance, and ultimately better patient selection for active surveillance or therapy. Furthermore, avoiding biopsy for patients with a normal MRI may limit the over-detection of indolent tumors. With these advantages in mind, our institutional decision was to adopt MRI targeted biopsy. In order to address the optimal MRI targeted method, visual or software co-registration, we PROspectively compare targeted biopsy outcomes between MRI-US-FUSion and visual estimation targeting (PROFUS trial) (5). Prospective targeted biopsy was performed in 125 consecutive men with suspicious regions identified on prebiopsy 3-T MRI. Two MRI-ultrasound fusion targeted cores per target were performed by one operator using the Artemis/Profuse (Eigen, Grass Valley, CA) platform. Targets were then blinded, and a second operator took two visually targeted cores and a 12-core biopsy. We found that MRI-US-fusion biopsy was more often histologically informative than visual targeting but did not increase cancer detection. A trend toward increased detection with fusion biopsy was observed across all study subsets, potentially suggesting a need for a larger study size.
Operator reproducibility
Learning curve
While MRI-US-fusion-targeted biopsy allows for improved targeting and detection of clinically significant prostate cancer, a concerning number of clinically significant cancers is still missed, as a result, most studies support the concurrent use of systematic biopsy. Previously reports have demonstrated that there is a learning curve associated with prostate MRI interpretation, both in terms of detection and staging (6,7). An additional study demonstrated that cancer detection rates improved from 27% to 63% over a 2 year period when evaluating the learning curve associated with MRI-US fusion targeted trans-perineal prostate biopsy (7). We recently reviewed our institutional learning curve of 1400 MRI-US-fusion targeted biopsies and found an increased cancer detection rate with increasing institutional operator experience (Meng, personal correspondence).
Program development and expansion at NYU
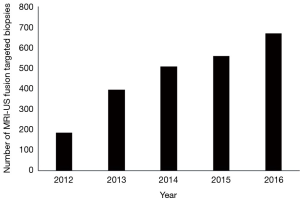
In May of 2012 we adopted the software co-registration MRI-US fusion targeted biopsy paradigm and since have performed more 1900 biopsies using this technique (Figure 1). Our biopsy pathway ensures that all men obtain a pre-biopsy MRI when feasible. All biopsies are performed by one of 5 urologic oncologists with experience in MRI-US-fusion biopsies. We have used the Artemis/Profuse (Eigen, Grass Valley, CA) fusion platform in which the biopsy is performed during an outpatient clinic visit, with a workflow comparable to that of a standard systematic trans-rectal ultrasound-guided biopsy (e.g., similar approach to analgesia as well as prevention of bleeding and infection; no catheterization).
Outcomes
Overall
In 2016, we reported the outcomes of our first 800 MRI-US fusion targeted biopsies (3). After exclusions, 601 men were included in the analysis. We found MRI-US fusion targeted biopsies detected fewer Gleason score 6 prostate cancer (75 vs. 121; P<0.001) and more Gleason score ≥7 prostate cancers (158 vs. 117; P<0.001) than systematic biopsy. Higher MRI suspicions score was associated with higher detection of Gleason score ≥7 prostate cancer (P<0.001) but was not correlated with detection of GS 6 prostate cancer. Prediction of Gleason score ≥7 disease by MRI suspicion score varied according to biopsy indication. Compared to systematic biopsy, MRI-US fusion targeted biopsies identified more Gleason score ≥7 prostate cancers in men with no prior biopsy (88 vs. 72; P=0.012), in men with a prior negative biopsy (28 vs. 16; P=0.010), and in men with a prior cancer diagnosis (42 vs. 29; P=0.043). MRI-US fusion targeted biopsies detected fewer Gleason score 6 prostate cancers in men with no prior biopsy (32 vs. 60; P<0.001) and men with prior cancer (30 vs. 46; P=0.034).
Each biopsy indication
Clinical applications of pre-biopsy MRI prior to targeted biopsy
The Prostate Imaging Reporting and Data System version 2 (PI-RADS v2) provides guidance for the performance of prostate MRI (PI-RADS v2) (8). PI-RADS v2 indicates that examinations should include T2-weighted imaging, diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) imaging. DWI should be performed using a high b-value in the range of 1,400-2,000 sec/mm2 and with reconstruction of apparent diffusion coefficient (ADC) maps. DCE should be performed with a temporal resolution of at least 10 seconds. PI-RADS v2 also provides guidance in the interpretation and reporting of prostate MRI examinations. This approach entails characterizing each detected lesion’s suspicion for clinically significant cancer using a 1–5 scale.
Prostate MRI provides high diagnostic performance for the detection of clinically significant cancer and assists disease localization and risk stratification. A recent pooled data meta-analysis assessing the performance of prostate MRI in prostate cancer detection showed a specificity of 88%, sensitivity of 74%, with a negative predictive value of 65% to 94% (9). A separate study demonstrated the PI-RADS v2 score to be highly associated with tumor significance (10).
Previous negative biopsy—finding missed disease
In men with a previous negative biopsy who are undergo multiple repeat systematic biopsies, clinically significant cancer is found at each sampling round (11). Given the continued likelihood of cancer detection even by the fifth systematic biopsy, MRI-US-fusion targeted biopsy may be able to selectively identify those men with clinically significant cancer and limit the need for repeated biopsy. In order to address the utility of MRI-US-fusion biopsy in men with a previous negative biopsy, we evaluated 210 men presenting to our institution for prostate biopsy with ≥1 prior negative biopsy underwent MRI followed by MRI-US-fusion targeted biopsy and systematic biopsy (12). Forty-seven (29%) of 161 men meeting inclusion criteria were found to have prostate cancer. MRI-US-fusion targeted biopsy and systematic biopsy had overall cancer detection rates of 21.7% and 18.6% (P=0.36), respectively, and cancer detection rates for Gleason score ≥7 disease of 14.9% and 9.3% (P=0.02), respectively. Of 26 men with GS ≥7 disease, MRI-US-fusion targeted biopsy detected 24 (92.3%) whereas systematic detected 15 (57.7%; P<0.01). Using UCSF-CAPRA criteria, only 1 man was restratified from low risk to higher risk based on systematic results compared to MRI-US-fusion targeted biopsy alone. Among men with MRI suspicion score <4, 72% of detected cancers were low risk by UCSF-CAPRA criteria. We concluded that in men with previous negative biopsies and persistent suspicion of prostate cancer, systematic biopsy contributes little to the detection of GS ≥7 disease by MRI-US-fusion targeted biopsy, and avoidance of systematic biopsy bears consideration. Use of MRI and targeted biopsy in the setting of a prior negative biopsy is supported by the literature, but is contingent upon the availability of high quality MRI acquisition and interpretation (13). The American Urological Society and the Society of Abdominal Radiology Prostate Cancer Disease-Focused Panel consensus statement highlights the need for MRI to be interpreted using PIRADS v2 by radiologists experienced in prostate MRI interpretation and for biopsy to be performed by urologists experienced in performing MRI-targeted biopsies (13).
No previous biopsy—goal of finding lethal disease while missing non-lethal disease, reduction of over-detection
Prebiopsy MRI may improve the detection of high risk cancer in men who present for a first prostate biopsy while at the same time decrease the detection of indolent, potentially avoiding nonlethal cancers, ultimately reducing the over-detection of the disease. In order to investigate the clinical outcomes of those men with no previous biopsy, we reviewed 452 consecutive men who underwent prebiopsy MRI followed by MRI-US-fusion targeted biopsy and systematic biopsy at our institution between June 2012 and June 2015 (14). Prostate cancer was detected in 207 of 382 men (54.2%). The cancer detection rate of systematic biopsy and MRI-US-fusion targeted biopsy was 49.2% and 43.5%, respectively (P=0.006). MRI-US-fusion targeted biopsy detected more Gleason score 7 or greater cancers than systematic biopsy (117 of 132 or 88.6% vs. 102 of 132 or 77.3%, P=0.037). Of 41 cancers detected by systematic biopsy but not by MRI-US-fusion targeted biopsy 34 (82.9%) demonstrated Gleason 6 disease, and 26 (63.4%) and 34 (82.9%) were clinically insignificant by Epstein criteria and a UCSF CAPRA (University of California-San Francisco-Cancer of the Prostate Risk Assessment) score of 2 or less, respectively. We concluded that men presenting for primary prostate biopsy MRI-US-fusion targeted biopsy detects more high grade cancers than systematic biopsy. Most cancers detected by systematic biopsy and not by MRI-US-fusion targeted biopsy are at clinically low risk. Prebiopsy MRI followed by MRI-US-fusion targeted biopsy decreases the detection of low risk cancers while significantly improving the detection and risk stratification of high grade disease.
Use in surveillance program—localizing dominant disease, accurate classification of disease risk
One potential role for MRI is in the assessment and monitoring of men with low risk prostate cancer on surveillance. Although MRI cannot likely improve the oncologic outcomes of surveillance greatly, it may afford a tool for better baseline risk stratification, resulting secondarily in better selection of candidates for surveillance, requiring fewer follow-up biopsies. Several critical observations have been using MRI and fusion targeted biopsy with known cancer to date. First, MRI-US-fusion targeted biopsy increases the detection of occult high grade disease. Second, systematic biopsy uniquely identified a significant number of men with occult high grade disease not identified by MRI targeted biopsy, suggesting a need for both in maximizing biopsy information (15). This most likely reflects the high prevalence of small volumes of pattern 4 in this population. Lastly, MRI suspicion score predicts the likelihood of upgrade, particularly among those with significant upgrade to dominant Gleason pattern 4 (3,15). With these findings in mind, the role of MRI in surveillance remains to be determined. It is critical to establish its impact, in baseline risk assessment and in monitoring, to justify the substantial cost.
Integration in practice
When do we use prebiopsy MRI?
Many potential practical barriers exist to performing routine pre-biopsy MRI in the large populations considered for prostate biopsy. Since our adoption of MRI-US-fusion targeted biopsy for men at risk for prostate cancer in 2012, 98.9% or our patients obtained a pre-biopsy MRI (16). This high percentage indicates our ability to successfully implement routine pre-biopsy MRI followed by MRI-US-fusion targeted biopsy in a high-volume tertiary center. Reasons that pre-biopsy MRI was not performed in 17 patients were: contraindication due to the presence of a cardiac pacemaker (n=8), lack of insurance carrier (n=3), contraindication due to shrapnel (n=2), claustrophobia (n=2), and physician preference (n=2; both in the original month of implementation of routine pre-biopsy MRI in June 2012) (16).
Pre-biopsy risk stratification—do we biopsy everyone?
Advances in prostate MRI have prompted its use before prostate biopsy for disease evaluation in multiple clinical scenarios. In those men with a normal MRI, systematic biopsy may possibly be avoided. We determined the rates of disease detection on systematic biopsy with a negative MRI which could enhance decision-making capability for men considering prostate biopsy (17). In our cohort of 75 patients, men with no previous biopsy, men with previously negative biopsy and men enrolled in active surveillance protocols, the overall cancer detection rates were 18.7%, 13.8%, 8.0% and 38.1%, respectively, and the detection rates for Gleason score ≥7 cancer were 1.3%, 0%, 4.0% and 0%, respectively. We found a negative prebiopsy MRI confers an overall negative predictive value of 82% on 12-core biopsy for all cancer and 98% for Gleason score ≥7 cancer (17).
More recently, nomograms have been enhanced to incorporate MRI findings to predict both overall and clinically significant cancer risk, which allows for counseling men on the need for biopsy. These nomograms have substantially improved predictive accuracy for both endpoints, even in diverse populations as well as in patients with no prior biopsy or with a prior negative biopsy (18).
Role of biomarkers
New biomarkers, such as kallikrein panels (4K Score and Prostate Health Index) and urine biomarkers (PCA3 and TMPRSS2-ERG), may improve further upon existing prostate cancer screening, detection, and risk assessment tools. The implementation of these biomarkers as secondary tools in conjunction with MRI could improve specificity markedly, sparing as many as half of men with an elevated PSA the need to undergo biopsy. We evaluated whether a combination of PCA3 and MRI suspicion score could further optimize detection of prostate cancer on MRI fusion-targeted biopsy among men with no history of biopsy (19). Our results showed that PCA3 <35 demonstrates a high negative predictive value among MRI suspicion score 2-3. However, in the case of high-suspicion MRI, PCA3 was not associated with cancer detection on MRI-US-fusion targeted biopsy, adding little to cancer diagnosis. By biopsying men with a MRI suspicion score of 4-5 and obtaining PCA3 on men with a MRI suspicion score of 2-3, followed by biopsy only in men with PCA3 score >35, 36.1% of biopsies would be avoided, and 4.9% of GS ≥3 + 4 cancers would have been missed (19). Other ancillary markers may help select patients with a negative/low-suspicion MRI for systematic biopsy.
Role of repeat biopsy
Patients receiving a PI-RADS assessment category of 3 to 5 warrant repeat biopsy with image guided targeting (20). At least 2 targeted cores should be obtained from each MRI defined target. Each man must be individually assessed as to whether a concurrent systematic sampling is warranted. Performing solely targeted biopsy should only be considered once quality assurance efforts have validated the performance of prostate MRI interpretations with results consistent with the published literature (20). In patients with negative or low suspicion MRI (PI-RADS assessment category of 1 or 2, respectively), other ancillary markers may be of value in identifying patients warranting repeat systematic biopsy. If a repeat biopsy is deferred on the basis of MRI findings, then continued clinical and laboratory follow-up is advised and consideration should be given to incorporating repeat MRI in this diagnostic surveillance regimen. Among men with very high suspicion (PI-RADS 5) and a negative targeted biopsy, early consideration should be given to repeat biopsy.
Conclusions
Prostate MRI and MRI-US-fusion targeted biopsy is employed in the detection of prostate cancer with the goals of reducing the detection of clinically insignificant disease, maximizing the detection of clinically significant cancer, along with better assessment of disease size, grade, and location. With these advantages, we have adopted this into our institutional paradigm for prostate cancer detection. Our outcomes support the clinical applications of MRI-US-fusion targeted biopsy in men who have never been biopsied before, those with a prior negative biopsy, and those with low risk disease considering active surveillance. Use of prebiopsy MRI, in conjunction with traditional clinical parameters and secondary biomarkers, may allow more accurate risk stratification and assessment of need for prostate biopsy.
Acknowledgements
Funding: Joseph and Diane Steinberg Charitable Trust.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bjurlin MA, Meng X, Le Nobin J, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol 2014;192:648-58. [Crossref] [PubMed]
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of Mr/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390-7. [Crossref] [PubMed]
- Meng X, Rosenkrantz AB, Mendhiratta N, et al. Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion-targeted prostate biopsy outcomes. Eur Urol 2016;69:512-7. [Crossref] [PubMed]
- Porpiglia F, Manfredi M, Mele F, et al. Diagnostic Pathway with Multiparametric Magnetic Resonance Imaging Versus Standard Pathway: Results from a Randomized Prospective Study in Biopsy-naïve Patients with Suspected Prostate Cancer. Eur Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol 2014;66:343-51. [Crossref] [PubMed]
- Rosenkrantz AB, Ayoola A, Hoffman D, et al. The learning curve in prostate MRI interpretation: Self-Directed learning versus continual reader feedback. AJR Am J Roentgenol 2017;208:W92-W100. [Crossref] [PubMed]
- Latchamsetty KC, Borden LS, Porter CR, et al. Experience improves staging accuracy of endorectal magnetic resonance imaging in prostate cancer: what is the learning curve? Can J Urol 2007;14:3429-34. [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- de Rooij M, Hamoen EH, Futterer JJ, et al. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014;202:343-51. [Crossref] [PubMed]
- Martorana E, Pirola GM, Scialpi M, et al. Lesion volume predicts prostate cancer risk and aggressiveness: validation of its value alone and matched with prostate imaging reporting and data system score. BJU Int 2017;120:92-103. [PubMed]
- Abraham NE, Mendhiratta N, Taneja SS. Patterns of repeat prostate biopsy in contemporary clinical practice. J Urol 2015;193:1178-84. [Crossref] [PubMed]
- Mendhiratta N, Meng X, Rosenkrantz AB, et al. Prebiopsy MRI and MRI-ultrasound Fusion-targeted Prostate Biopsy in Men With Previous Negative Biopsies: Impact on Repeat Biopsy Strategies. Urology 2015;86:1192-8. [Crossref] [PubMed]
- Rosenkrantz AB, Verma S, Choyke P, et al. Prostate Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Patients with a Prior Negative Biopsy: A Consensus Statement by AUA and SAR. J Urol 2016;196:1613-18. [Crossref] [PubMed]
- Mendhiratta N, Rosenkrantz AB, Meng X, et al. Magnetic resonance Imaging-Ultrasound fusion targeted prostate biopsy in a consecutive cohort of men with no previous biopsy: reduction of over detection through improved risk stratification. J Urol 2015;194:1601-6. [Crossref] [PubMed]
- Tran GN, Leapman MS, Nguyen HG, et al. Magnetic Resonance Imaging-Ultrasound Fusion Biopsy During Prostate Cancer Active Surveillance. Eur Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Rosenkrantz AB, Lepor H, Huang WC, et al. Practical barriers to obtaining Pre-Biopsy prostate MRI: assessment in over 1,500 consecutive men undergoing prostate biopsy in a single urologic practice. Urol Int 2016;97:247-8. [Crossref] [PubMed]
- Wysock JS, Mendhiratta N, Zattoni F, et al. Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12-core biopsy results. BJU Int 2016;118:515-20. [Crossref] [PubMed]
- Bjurlin M, Wysock J, Sakar S, et al. A pre-biopsy nomogram for prediction of the risk of gleason score=7 prostate cancer on combined MRI-US fusion targeted and systematic prostate biopsy among men with no previous biopsy. Journal of Urology 2016;195:E701. [Crossref]
- Fenstermaker M, Mendhiratta N, Bjurlin MA, et al. Risk stratification by urinary prostate cancer gene 3 testing before magnetic resonance Imaging-Ultrasound fusion-targeted prostate biopsy among men with no history of biopsy. Urology 2017;99:174-9. [Crossref] [PubMed]
- Rosenkrantz AB, Verma S, Choyke P, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol 2016;196:1613-8. [Crossref] [PubMed]