Sperm DNA fragmentation for the evaluation of male infertility: clinical algorithms
Dear Editor ,
We acclaim the commentary written by Mirzazadeh and Sadri-Ardekani on our article addressing the clinical utility of sperm DNA fragmentation testing by Agarwal et al. (1). The authors’ contribution is a valuable addition to the clinical debate surrounding this important topic. The authors have not only acknowledged the role of SDF testing in the evaluation of infertile men, but also offered sound algorithms that should further clarify the indications for SDF testing in patients with varicocele, unexplained infertility, recurrent miscarriage, recurrent intrauterine insemination (IUI) and in vitro fertilization (IVF) failure, and recurrent miscarriage after intracytoplasmic sperm injection (ICSI). We thank the authors for their useful addition, and we aim to further elucidate their views in this reply and suggest some changes to their proposed algorithms.
Mirzazadeh and Sadri-Ardekani admitted that despite the current recommendations by the American Urological Association (AUA) (2), American Society for Reproductive Medicine (ASRM) (3) and European Association of Urology (EAU) (4) which generally endorse against the routine use of SDF in the clinical evaluation of infertile men, they still believe that SDF testing plays a significant role in clinical decision making. In fact, clinical recommendations are looked at as best practice statements that aim to guide clinicians on evidence-based approach for management. In this sense, with further research and development into a particular field of medicine, a constant update to the clinical recommendations is periodically required. To this end, numerous studies exploring the effects of SDF on male fertility have been published lately. Good quality studies reporting a significant detrimental relationship between SDF and clinical varicocele (5-7), unexplained infertility (8,9), and outcomes of assisted reproductive treatments (ART) (10-12) were published recently highlighting the need for further updates in the clinical recommendations of the abovementioned societies.
Mirzazadeh and Sadri-Ardekani have pointed out that our practice recommendations contained extensive information on the various laboratory tests used for SDF measurement suggesting that such a discussion may be more applicable for laboratory specialists than clinicians. We disagree with their view on this point as we feel that this information is crucial for clinicians interpreting the SDF results. The main preventing barring widespread adoption of the SDF test is the fact that a variety of SDF test modalities exist results in a misunderstanding of the exact DNA damage that each method tests and the difficulty in recognizing an agreed upon threshold for the SDF abnormality. Therefore, the clinician ordering the SDF test needs to be well acquainted with the method that was performed on his patient to accurately interpret the test result.
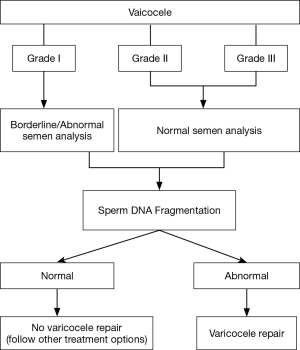
The authors have provided an algorithm on the clinical utility of SDF in varicocele patients. While we agree with the authors that this should clarify the intended message, we have modified their algorithm by incorporating the clinical grade of the varicocele (Figure 1). Perhaps there isn’t a topic in the field of male infertility that was investigated more that varicocele (13). Clinical varicocele is recognized as the most common surgically reversible cause of male infertility. Despite that, clinicians are sometimes faced with equivocal scenarios especially related to lower grades of varicocele resulting in difficulty with clinical decision making. SDF is now believed to be significantly higher in patients with varicocele. A recently published meta-analysis showed that patients with varicoceles had significantly higher SDF than controls, with a mean difference of 9.84% (95% CI, 9.19 to 10.49; P<0.001) (14). This meta-analysis also showed that varicocelectomy improves sperm DNA integrity, with a mean difference of −3.37% (95% CI, −4.09 to −2.65; P<0.001). However, fewer studies have investigated SDF levels in lower grade varicocele. Sadek et al. (15) reported comparable measures of SDF in patients with varicocele grades 2 and 3, though they only detected a statistically significant reduction in SDF after varicocelectomy in patients with clinical grade 3 disease. On the other hand, Ni et al. (7) identified a significant reduction in the protamine-1/2 mRNA ratio in grade 3 varicocele and a significant decrease in DNA fragmentation in grades 2 and 3 diseases after surgery. Protamines 1 and 2 are nuclear proteins which replace histones during spermatogenesis; their ratio has been utilized as a diagnostic marker for male infertility and ART success (16). Additional research is needed to further elucidate the significance of SDF in grades 1/2 varicocele. Nonetheless, varicocelectomy, when appropriately indicated could result in beneficial outcome even in low-grade disease. Krishna Reddy et al. (17) investigated 482 infertile patients with varicocele and reported significant improvement in post-surgery semen parameters among all three groups of varicocele and further revealed that natural pregnancies occurred equally in lower grade varicocele compared with grade 3 varicocele. Therefore, with this updated algorithm we recommend ordering SDF testing in patients with clinical grade 1 varicocele with borderline/abnormal semen parameters and in patients with grades 2/3 varicocele with normal semen parameters. Finding a high SDF result should aid the clinician in recommending surgical ligation of the varicocele.
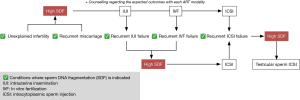
Mirzazadeh and Sadri-Ardekani were apparently in full agreement with the clinical utility of SDF in patients with unexplained infertility, recurrent miscarriage and recurrent failure or miscarriage after ART. However, they inadvertently mixed up between unexplained infertility and unexplained recurrent miscarriage which are two different clinical conditions. Based on the available evidence illustrating a significantly higher SDF in patients with unexplained infertility (8), recurrent miscarriage (18-20), recurrent IUI failure (21,22), recurrent IVF failure and recurrent miscarriage after ICSI (22,23), we developed another slight modification to the proposed algorithm by the authors where SDF is indicated in patients presenting with the above mentioned clinical scenarios (Figure 2). The SDF result shall then guide the practicing clinician in his/her decision so that patients with unexplained infertility and recurrent miscarriage are counseled about the possible outcomes of every ART they wish to perform, while patients with recurrent IUI and IVF failure are offered ICSI with or without physiologic sperm selection. Finally, patients with recurrent miscarriage after ICSI are offered testicular sperm retrieval + ICSI based on the evidence that testicular sperm have lower SDF levels and result in a better ICSI outcome (24).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Jarow J, Sigman M. The Optimal Evaluation of the Infertile Male: AUA Best Practice Statement. Available online: https://www.auanet.org/common/pdf/education/clinical-guidance/Male-Infertility-d.pdf
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015;103:e18-25. [Crossref] [PubMed]
- Jungwirth A, Diemer T, Dohle GR, et al. Guidelines on male infertility, European Association of Urology (EAU), Editor. 2015:Arnhem (The Netherlands).
- Telli O, Sarici H, Kabar M, et al. Does varicocelectomy affect DNA fragmentation in infertile patients? Indian J Urol 2015;31:116-9. [Crossref] [PubMed]
- Kadioglu TC, Aliyev E, Celtik M. Microscopic varicocelectomy significantly decreases the sperm DNA fragmentation index in patients with infertility. Biomed Res Int 2014;2014:695713. [Crossref] [PubMed]
- Ni K, Steger K, Yang H, et al. Sperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligation. J Urol 2014;192:170-6. [Crossref] [PubMed]
- Oleszczuk K, Augustinsson L, Bayat N, et al. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013;1:357-60. [Crossref] [PubMed]
- Leach M, Aitken RJ, Sacks G. Sperm DNA fragmentation abnormalities in men from couples with a history of recurrent miscarriage. Aust N Z J Obstet Gynaecol 2015;55:379-83. [Crossref] [PubMed]
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. [Crossref] [PubMed]
- Jin J, Pan C, Fei Q, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril 2015;103:910-6. [Crossref] [PubMed]
- Zhao J, Zhang Q, Wang Y, et al. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014;102:998-1005.e8. [Crossref] [PubMed]
- Majzoub A, Esteves SC, Gosálvez J, et al. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl 2016;18:205-12. [Crossref] [PubMed]
- Wang YJ, Zhang RQ, Lin YJ, et al. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online 2012;25:307-14. [Crossref] [PubMed]
- Sadek A, Almohamdy AS, Zaki A, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril 2011;95:1705-8. [Crossref] [PubMed]
- Aoki VW, Moskovtsev SI, Willis J, et al. DNA integrity is compromised in protamine-deficient human sperm. J Androl 2005;26:741-8. [Crossref] [PubMed]
- Krishna Reddy SV, Shaik AB, Sailaja S, et al. Outcome of Varicocelectomy with Different Degrees of Clinical Varicocele in Infertile Male. Advances in Andrology 2015:9.
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2:76-83. [PubMed]
- Khadem N, Poorhoseyni A, Jalali M, et al. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia 2014;46:126-30. [Crossref] [PubMed]
- Absalan F, Ghannadi A, Kazerooni M, et al. Value of sperm chromatin dispersion test in couples with unexplained recurrent abortion. J Assist Reprod Genet 2012;29:11-4. [Crossref] [PubMed]
- Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122-8. [Crossref] [PubMed]
- Bungum M, Humaidan P, Spano M, et al. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod 2004;19:1401-8. [Crossref] [PubMed]
- Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 2009;30:219-29. [Crossref] [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]