Magnetic resonance imaging detection of prostate cancer in men with previous negative prostate biopsy
Introduction
Current methods of prostate cancer (PCa) screening and diagnosis have come under scrutiny because of their lack of diagnostic accuracy and performance. Historically, the current standard of care for prostate biopsy has been a 10–12 core transrectal ultrasound guided systematic biopsy (SB). The technique is lacking in diagnostic accuracy because of sampling error, and it’s estimated that well less than 1% of the prostate is sampled during a prostate biopsy. Men with a persistent suspicion of PCa with one or more negative SB represents a diagnostic dilemma for urologists. Prostate multiparametric MRI (mpMRI) has been useful in this population by identifying suspicious prostate lesions, often in areas under-sampled by the SB. Currently the recommended mpMRI of the prostate consists of T1-weighted, high resolution T2-weighted images and at least 2 functional MRI techniques (1,2).
Targeted biopsy (TB) of suspicious lesions seen on MRI can be done cognitively under US guidance, performed in-gantry in a MRI suite, or using a MR/US fusion biopsy platform (3). Although debatable, under most circumstances concurrent SB continues to be performed at the time of TB biopsy given the occasional diagnosis of Gleason score ≥7 cancers uniquely identified to the SB alone (4). Many authors have found that MR fusion biopsy (which consists of TB + SB) is able to detect more clinically significant PCa (csPCa) than either modality alone, while many definitions of csPCa exist in the literature (5).
A growing body of literature has been in support of MR/US fusion biopsy, particularly in patients with a prior negative SB. Although the increased cost of this technology has been a concern, fusion biopsy technology has generally been gaining acceptance. In a recent survey sent to members of Society of Urological Oncology, Endourological Society, European Association of Urology, 85.7% of respondents use prostate MRI in the practice and 63% use MR/US TB (6). Quality assurance is critical with adoption of this technology, since an institutional learning curve is associated with adoption of this technology (7).
Methods
A PubMed and Web of Science database searches of peer reviewed literature using the following search terms (I) “magnetic resonance imaging” AND (“previous” or “prior” or “failed”) and “biopsy” and “PCa”; (II) “magnetic resonance imaging” and “PCa” and “biomarkers.” Over 1,100 articles were identified in the last 10 years and abstracts were screened for relevance. If relevance could not be determined by the abstract, then the full text was reviewed. Studies that included only biopsy-naïve patients were excluded from this review. Using relevance and quality of study design as criteria for inclusion, a total of 51 seminal articles on the topic of MR/US fusion TB in patients with a prior SB were selected by two authors by consensus to be included in this review
Results
How does MR-US fusion TB with concurrent SB compare to SB alone in patients with a prior negative biopsy?
Improved cancer detection rate (CDR), particularly CDR for csPCa, is frequently cited a rationale for use of MR fusion biopsy. The reported CDR for csPCa in men with a prior negative biopsy can range 20–40% and is lower compared to detection rates in biopsy-naive patients (8,9). The attenuation in CDR is likely due to the reduced prevalence of higher volume disease in patients with a prior biopsy (10). Thus far, the vast majority of studies that favor TB + SB over SB in the prior negative biopsy cohort have used a retrospective or prospective cohort design (3,7,10-22) (Table 1). Currently, there are no published randomized controlled trials comparing the performance of TB + SB and SB in patients with at least one prior negative biopsy.
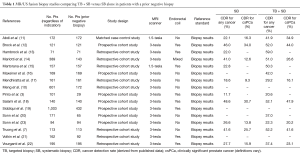
Full table
These cohort studies found that the TB + SB had a higher CDR for csPCa (typically defined as Gleason score ≥7) compared to the SB biopsy alone. The implication is that the TB is able to accurately sample cancerous areas of the prostate that are frequently missed on SB (20). In addition to increasing CDR, an additional advantage of fusion biopsy is the potential to reduce over detection of clinically insignificant cancers. Siddiqui et al. reported MR/US fusion biopsy data on 1,003 men of which almost half were patients with prior negative biopsy. They found TB diagnosed 30% more high risk and 17% less low risk cancer than SB alone. In their cohort, there were significantly more anterior lesions seen in men with prior negative biopsy (19). Another group reported on their 1,042 patients who had undergone fusion biopsy, in which 324 had a prior negative biopsy. They found the combination of TB + SB was superior to either modality alone, with 61% of the men having cancer detected on TB + SB having Gleason ≥7, versus 50% with SB alone (24). A study from the UK evaluated 54 men with at least one prior negative TRUS biopsy. All men had an mpMRI and then underwent a transperineal systematic prostate biopsies to determine the imaging accuracy in identifying cancer. They found sensitivities between 76–90% and negative predictive values between 75–95% for identify clinically significant cancer depending on the different definitions used (25). Researchers from UCLA previously reported on 105 patients with prior negative biopsies who went on the have a MRI/US fusion biopsy. They found that 91% of men with cancer found on TB had clinically significant cancer compared to only 54% with SB. MRI suspicion score was the most powerful predictor of identifying clinically significant cancer and independent of the number of prior negative biopsies (23).
Two separate meta-analyses found that the overall CDR of any PCa between TB + SB and SB did not significantly differ, but more csPCa and fewer insignificant cancers were detected with TB + SB. Although TB + SB may improve detection of significant PCa in men with previous negative biopsy, this has not consistently been shown in biopsy naïve men (8,26). A meta-analysis comparing MR fusion biopsy, SB, and perineal saturation biopsy suggested that fusion biopsy yielded the highest CDR and the lower number of cores needed to achieve cancer detection (27). According to a consensus statement by American Urological Association and the Society of Abdominal Radiology, use of MR/US guided biopsy after a prior negative biopsy is supported by the literature, but is contingent upon the availability and quality of MRI acquisition and interpretation (4).
These studies which report the CDR between TB + SB to SB in patients with prior negative biopsies can be difficult to compare directly because some only include patients with MRI visible lesions while others include patients with both positive and negative MRIs. Another limitation is the performance of concurrent SB at the time of MR/US fusion biopsy is used as a surrogate for SB alone, the gold standard reference remains final whole mount pathology, but for obvious reasons that is not always possible. A non-randomized study utilized a matched cohort approach comparing MR TB versus SB alone without prior MRI to circumvent the potential bias of knowing where suspicious lesions were seen on mpMRI. The study found that MR TB diagnosed twice as many cases of clinically significant cancer without an increase in the diagnosis of clinically insignificant cancers (11).
A highly discussed topic regarding fusion biopsy studies is what actually constitute significant disease found on both MRI and fusion biopsy. Many studies apply definitions of csPCa typically used for systematic biopsies, but the most accurate identification of clinical significance is through comparison of prostatectomy pathology to that of the cancer detected by SB and fusion biopsy (28). One study suggested that the MRI-estimated lesion volume is associated with higher Gleason and stage at radical prostatectomy (15). The implication is that mpMRI is able to provide a better estimation of tumor volume and more accurately characterize the cancer.
Incorporating MRI into clinical practice does add addition cost; however, these additional costs can be offset by the reduction of costs associated with false positive biopsies and underestimation of tumor aggressiveness. In one study, a decision tree model based on Medicare reimbursements to compare the costs of TRUS biopsy versus fusion biopsy in men with prior negative biopsy (29). The MRI-based approach was most cost-effective for low risk patients (<30% probability of having cancer) and the cost savings found in the MRI group is related to the number of men which have negative MRIs and thus are spared a repeat biopsy. In another cost-effectiveness analysis, the cost of MRI/US fusion biopsy was comparable to SB. In this analysis, the costs were similar, but fusion biopsy led to an improvement in quality of life compared to SB due to the reduction in over diagnosis and subsequent treatment (30).
Anterior and apical tumors
An advantage of fusion biopsy in patients with prior negative biopsy is the ability of MRI to identify suspicious lesions in areas not normally sampled by SB—specifically the anterior and apical parts of the prostate and thus the benefit of fusion biopsy is particularly accentuated in the prior negative biopsy cohort (17). A frequent observation is that csPCa is more frequently detected in the anterior prostate in the prior negative biopsy setting (31). One study evaluated the performance of fusion biopsy in the distal apical prostate, defined as the distal 6 mm apical portion of the prostate starting from the distal most visualized part of the prostate. They found that 80% of suspicious lesions in this distal apical region were positive for cancer, and 33% of these patients had Gleason upgrading because of this TB (32). Another study found that among patients with a prior negative biopsy who underwent MR fusion biopsy, significantly more men with PCa identified on TB had anterior or apical lesions. In addition, of these patients that went on to have a radical prostatectomy, 86% had tumors at either the anterior or apex of the prostate (33). A different study concluded that in men with prior negative biopsies, not only were anterior lesions more common 70% vs. 30% peripheral zone, but that the majority, 93%, was intermediate-high risk (34).
MR fusion biopsy allows for more accurate characterization of the overall tumor burden, in part by its identification of anterior lesions. In one study, anterior lesions found on MRI with tumor involvement were 112% longer in the TB cores than in the concurrent SB cores (3.7 vs. 1.6 mm) (21). Racial differences have also been reported showing that African-American men with prior biopsy were twice as likely compared to white/other races to harbor cancer in the anterior prostate, suggesting that these men benefit even more from a MRI-based approach (35). Biopsy of anterior lesions can also be performed using transperineal approach. MR/US guided transperineal saturation biopsy has also been previously described with detection rates similar to transrectal approaches (36). In a comparative analysis of transperineal saturation biopsy versus FB after mpMRI, both methods had a similar detection rate for Gleason score ≥7 cancers, but FB reduced overdetection of Gleason 3+3 cancers (37). Furthermore, a major advantage of FB over transperineal saturation biopsy is that FB does not require general anesthesia and can be performed in the clinic setting. Data is sparse regarding use of a transurethral biopsy approach after MRI. Recently, a study of a novel technique using transurethral resection to diagnose anterior located tumors identified on MRI allowed detection of Gleason score ≥7 cancers in 13/16 patients (38).
MRI reporting
Due to significant variability in inter-observer MRI reads, PI-RADS Version 2 was recently developed in an effort to standardize image reporting by radiologists (39). In a recent meta-analysis, the pooled sensitivity and specificity for PIRADS scoring was 0.78 and 0.79, respectively, (40) which has been a substantial improvement over that of early studies (41,42). One study reported and improved AUC of as high as 0.86 for MRI/real-time elastography fusion in patients with prior negative biopsies (12). Currently, most centers will perform targeted biopsies of lesions with a PIRADS score of 3–5, but knowing your own institution’s biopsy performance and accuracy is paramount in guiding recommendations (4). A consensus statement also states at least 2 cores from each MRI target is recommended in patients undergoing FB after a prior negative biopsy (4). However, some groups have reported a higher CDR with an increasing number of cores obtained using TB in this patient population (7). CDR is variable across studies and is influenced by a variety of factors, including patient selection, accuracy of MRI reads, and targeting errors (43).
How does mpMRI compare to other risk stratification tools?
Currently, only a limited number of studies have directly compared the performance of mpMRI against clinical variables (PSA, DRE, PSAD), biomarkers, or nomograms. A PSAD of ≤0.2 was associated with low detection of Gleason score ≥7 in men with negative MRI and in men with equivocal imaging and may be useful for determining which men should opt for surveillance rather than repeat biopsy (44). In one study, the ability of mpMRI was directly compared against PHI and PCA3 for predicting biopsy outcome in the repeat biopsy setting (45). The combination of MRI + base model (DRE and PSA) had an AUC of 0.936 for predicting the presence of cancer at biopsy and was superior to PHI + base model or PCA3 + base model. Another study evaluated whether PCA3 could be combined with PIRADS version 1 score to predict a positive biopsy at the time of fusion biopsy after a previous negative SB. When PCA3 score is treated as a binary variable (PCA3 score >80), both PCA3 score and PIRADS score ≥4 predicted a positive repeat SB in a multivariate model (46). A small randomized study compared the use of PCA3 alone and PCA3+ FB in men with prior negative biopsy and found a marginal increase in the AUC (0.85 vs. 0.82) in the PCA+ FB arm (47). The current NCCN guidelines state that the following tests can be considered in patients thought to be at a higher risk despite a negative biopsy to inform the decision about performing a repeat biopsy: %f PSA, 4Kscore, PHI, PCA3, or ConfirmMDx.
A recent study found that mpMRI findings correlated well with Gleason score, but was not able to detect all aggressive PCas as defined by the Prolaris® CCP score. This study suggests that a combined molecular biomarker with radiomics (use of mpMRI-derived variables) in the future may provide the best sensitivity and specificity for significant disease (48). Whether these findings can be applied to men who have a prior negative biopsy undergoing fusion biopsy is unclear.
Conclusions
Patients who have a prior negative biopsy and persistent suspicion of PCa represent a diagnostic dilemma for urologists. When a suspicious lesion is found on mpMRI, TB has consistently demonstrated improvement in clinically significant cancer detection compared to a repeat SB. Importantly, TB allows for better sampling of difficult to reach tumors in the anterior and apex of the prostate. In addition, fusion biopsy may be more cost-effective in the long-term by reducing the number of ineffective SBs and improving quality of life.
While the advances in MRI fusion biopsy technology are promising and an exciting area of research, urologists should be mindful of potential limitations of mpMRI. The performance of TB relies solely on the ability of mpMRI to identify clinically significant cancer, but mpMRI can miss small but significant tumors, and it is for this reason that TB + SB offers the best diagnostic accuracy (49). The observed increased in clinically significant cancer detection has been consistently demonstrated across cohort studies despite wide variations in MRI protocols and operator technique. It is for this reason that all men with a prior negative biopsy and continued suspicion of PCa should strongly be encouraged to get a prostate mpMRI prior to a repeat biopsy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding--multiparametric MR imaging for detection and biopsy planning. Radiology 2011;259:162-72. [Crossref] [PubMed]
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012 Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186:1281-5. [Crossref] [PubMed]
- Rosenkrantz AB, Verma S, Choyke P, et al. Prostate Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Patients with a Prior Negative Biopsy: A Consensus Statement by AUA and SAR. J Urol 2016;196:1613-8. [Crossref] [PubMed]
- Ploussard G, Epstein JI, Montironi R, et al. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol 2011;60:291-303. [Crossref] [PubMed]
- Muthigi A, Sidana A, George AK, et al. Current beliefs and practice patterns among urologists regarding prostate magnetic resonance imaging and magnetic resonance-targeted biopsy. Urol Oncol 2017;35:32.e1-32.e7. [Crossref] [PubMed]
- Truong M, Weinberg E, Holleberg G, et al. Institutional Learning Curve Associated with Implementation of a MR/ US Fusion Biopsy Program using PI-RADS Version 2: Factors that Influence Success. Urology Practice 2017. [Epub ahead of print]. [Crossref]
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. [Crossref] [PubMed]
- Fütterer JJ, Verma S, Hambrock T, et al. High-risk prostate cancer: value of multi-modality 3T MRI-guided biopsies after previous negative biopsies. Abdom Imaging 2012;37:892-6. [Crossref] [PubMed]
- Meng X, Rosenkrantz AB, Mendhiratta N, et al. Relationship Between Prebiopsy Multiparametric Magnetic Resonance Imaging (MRI), Biopsy Indication, and MRI-ultrasound Fusion-targeted Prostate Biopsy Outcomes. Eur Urol 2016;69:512-7. [Crossref] [PubMed]
- Abdi H, Zargar H, Goldenberg SL, et al. Multiparametric magnetic resonance imaging-targeted biopsy for the detection of prostate cancer in patients with prior negative biopsy results. Urol Oncol 2015;33:165.e1-7. [Crossref] [PubMed]
- Brock M, Loppenberg B, Roghmann F, et al. Impact of real-time elastography on magnetic resonance imaging/ultrasound fusion guided biopsy in patients with prior negative prostate biopsies. J Urol 2015;193:1191-7. [Crossref] [PubMed]
- Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol 2010;183:520-7. [Crossref] [PubMed]
- Mariotti GC, Costa DN, Pedrosa I, et al. Magnetic resonance/transrectal ultrasound fusion biopsy of the prostate compared to systematic 12-core biopsy for the diagnosis and characterization of prostate cancer: multi-institutional retrospective analysis of 389 patients. Urol Oncol 2016;34:416.e9-416.e14. [Crossref] [PubMed]
- Martorana E, Pirola GM, Scialpi M, et al. Lesion volume predicts prostate cancer risk and aggressiveness: validation of its value alone and matched with prostate imaging reporting and data system score. BJU Int 2017;120:92-103. [PubMed]
- Maxeiner A, Stephan C, Durmus T, et al. Added Value of Multiparametric Ultrasonography in Magnetic Resonance Imaging and Ultrasonography Fusion-guided Biopsy of the Prostate in Patients With Suspicion for Prostate Cancer. Urology 2015;86:108-14. [Crossref] [PubMed]
- Mendhiratta N, Meng X, Rosenkrantz AB, et al. Prebiopsy MRI and MRI-ultrasound Fusion-targeted Prostate Biopsy in Men With Previous Negative Biopsies: Impact on Repeat Biopsy Strategies. Urology 2015;86:1192-8. [Crossref] [PubMed]
- Salami SS, Ben-Levi E, Yaskiv O, et al. In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU Int 2015;115:562-70. [Crossref] [PubMed]
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390-7. [Crossref] [PubMed]
- Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol 2013;189:86-91. [Crossref] [PubMed]
- Volkin D, Turkbey B, Hoang AN, et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int 2014;114:E43-49. [Crossref] [PubMed]
- Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 2012;188:2152-7. [Crossref] [PubMed]
- Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol 2014;65:809-15. [Crossref] [PubMed]
- Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016;122:884-92. [Crossref] [PubMed]
- Abd-Alazeez M, Ahmed HU, Arya M, et al. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level--can it rule out clinically significant prostate cancer? Urol Oncol 2014;32:45.e17-22. [Crossref] [PubMed]
- Wu J, Ji A, Xie B, Wang X, et al. Is magnetic resonance/ultrasound fusion prostate biopsy better than systematic prostate biopsy? An updated meta- and trial sequential analysis. Oncotarget 2015;6:43571-80. [PubMed]
- Nelson AW, Harvey RC, Parker RA, et al. Repeat prostate biopsy strategies after initial negative biopsy: meta-regression comparing cancer detection of transperineal, transrectal saturation and MRI guided biopsy. PLoS One 2013;8:e57480. [Crossref] [PubMed]
- van Hove A, Savoie PH, Maurin C, et al. Comparison of image-guided targeted biopsies versus systematic randomized biopsies in the detection of prostate cancer: a systematic literature review of well-designed studies. World J Urol 2014;32:847-58. [Crossref] [PubMed]
- Lotan Y, Haddad AQ, Costa DN, et al. Decision analysis model comparing cost of multiparametric magnetic resonance imaging vs. repeat biopsy for detection of prostate cancer in men with prior negative findings on biopsy. Urol Oncol 2015;33:266.e9-16. [Crossref] [PubMed]
- de Rooij M, Crienen S, Witjes JA, et al. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol 2014;66:430-6. [Crossref] [PubMed]
- Zugor V, Kuhn R, Engelhard K, et al. The Value of Endorectal Magnetic Resonance Imaging of the Prostate in Improving the Detection of Anterior Prostate Cancer. Anticancer Res 2016;36:4279-83. [PubMed]
- Nix JW, Turkbey B, Hoang A, et al. Very distal apical prostate tumours: identification on multiparametric MRI at 3 Tesla. BJU Int 2012;110:E694-700. [Crossref] [PubMed]
- Lee SH, Chung MS, Kim JH, et al. Magnetic resonance imaging targeted biopsy in men with previously negative prostate biopsy results. J Endourol 2012;26:787-91. [Crossref] [PubMed]
- Schouten MG, Hoeks CM, Bomers JG, et al. Location of Prostate Cancers Determined by Multiparametric and MRI-Guided Biopsy in Patients With Elevated Prostate-Specific Antigen Level and at Least One Negative Transrectal Ultrasound-Guided Biopsy. AJR Am J Roentgenol 2015;205:57-63. [Crossref] [PubMed]
- Kongnyuy M, Sidana A, George AK, et al. The significance of anterior prostate lesions on multiparametric magnetic resonance imaging in African-American men. Urol Oncol 2016;34:254.e15-21. [Crossref] [PubMed]
- Hansen N, Patruno G, Wadhwa K, et al. Magnetic Resonance and Ultrasound Image Fusion Supported Transperineal Prostate Biopsy Using the Ginsburg Protocol: Technique, Learning Points, and Biopsy Results. Eur Urol 2016;70:332-40. [Crossref] [PubMed]
- Radtke JP, Kuru TH, Boxler S, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol 2015;193:87-94. [Crossref] [PubMed]
- Dason S, Allard CB, Wright I, et al. Transurethral Resection of the Prostate Biopsy of Suspected Anterior Prostate Cancers Identified by Multiparametric Magnetic Resonance Imaging: A Pilot Study of a Novel Technique. Urology 2016;91:129-35. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- Hamoen EH, de Rooij M, Witjes JA, et al. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol 2015;67:1112-21. [Crossref] [PubMed]
- Singh AK, Krieger A, Lattouf JB, et al. Patient selection determines the prostate cancer yield of dynamic contrast-enhanced magnetic resonance imaging-guided transrectal biopsies in a closed 3-Tesla scanner. BJU Int 2008;101:181-5. [PubMed]
- Lattouf JB, Grubb RL 3rd, Lee SJ, et al. Magnetic resonance imaging-directed transrectal ultrasonography-guided biopsies in patients at risk of prostate cancer. BJU Int 2007;99:1041-6. [Crossref] [PubMed]
- Cash H, Günzel K, Maxeiner A, et al. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: reasons for targeted biopsy failure. BJU Int 2016;118:35-43. [Crossref] [PubMed]
- Hansen NL, Barrett T, Koo B, et al. The influence of prostate-specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7-10 prostate cancer in a repeat biopsy setting. BJU Int 2017;119:724-30. [Crossref] [PubMed]
- Porpiglia F, Russo F, Manfredi M, et al. The roles of multiparametric magnetic resonance imaging, PCA3 and prostate health index-which is the best predictor of prostate cancer after a negative biopsy? J Urol 2014;192:60-6. [Crossref] [PubMed]
- De Luca S, Passera R, Cattaneo G, et al. High prostate cancer gene 3 (PCA3) scores are associated with elevated Prostate Imaging Reporting and Data System (PI-RADS) grade and biopsy Gleason score, at magnetic resonance imaging/ultrasonography fusion software-based targeted prostate biopsy after a previous negative standard biopsy. BJU Int 2016;118:723-30. [Crossref] [PubMed]
- Sciarra A, Panebianco V, Cattarino S, et al. Multiparametric magnetic resonance imaging of the prostate can improve the predictive value of the urinary prostate cancer antigen 3 test in patients with elevated prostate-specific antigen levels and a previous negative biopsy. BJU Int 2012;110:1661-5. [Crossref] [PubMed]
- Renard-Penna R, Cancel-Tassin G, Comperat E, et al. Multiparametric Magnetic Resonance Imaging Predicts Postoperative Pathology but Misses Aggressive Prostate Cancers as Assessed by Cell Cycle Progression Score. J Urol 2015;194:1617-23. [Crossref] [PubMed]
- Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011;186:1818-24. [Crossref] [PubMed]


