DNA fragmentation and the ultimate success of a pregnancy
Two very recent comprehensive reviews on sperm DNA fragmentation tests (1,2) have reopened the debate over their usefulness in improving pregnancy outcome.
In this regards, two considerations should be disentangled. First, spermatozoa are not simply carriers of paternal chromosomes, but play a role beyond fertilization. For instance, the spermatozoon transcribes genes critical for early embryonic development, inferring that integrity of sperm genome is essential for a successful gestation. Second, if sperm factors play a role in early embryonic development, are sperm DNA integrity tests useful as diagnostic and prognostic markers, especially in the context of recurrent pregnancy loss (RPL) (3) ?
The concept that sperm quality influences the success of a pregnancy is far from new but still subject of ongoing controversy: In the 1950s, Joel (4) linked RPL in five women to oligozoospermia in the partner, whereas Macleod and Gold (5) observed that semen characteristics were poorer in the partners of subjects with repeated abortions than in those of fertile couples. Then, in the 1960s, Furuhjelm et al. claimed that fathers of children who died in the perinatal period had “a decreased concentration of spermatozoa and an increased percentage of morphologically abnormal cells” in their semen (6). In a controlled investigation, they also showed “a statistically highly significant increase in the percentage of abnormal spermatozoa” in the male partners of women whose pregnancies had ended in a spontaneous miscarriage (7). Contradicting these findings, some twenty years later Homonnai et al. (8) concluded “sperm concentration was significantly higher in the repeated and habitual abortion groups with a tendency to polyzoospermia”.
Explaining, at least in part, these contradictory early results is the notion that RPL is a multifactorial disorder caused by a multitude of factors, including uterine anomalies, hormonal imbalance, autoimmune diseases, thrombophilia, and free radical imbalance (9,10). Consequently, it has become commonplace to classify RPL as either “unexplained” or “idiopathic” miscarriage and, presumably, “explained” recurrent miscarriage. Yet, every diagnostic test currently in clinical practice lacks specificity, meaning that many women with normal pregnancies also test positive. Nevertheless, it remains standard practice to label a “positive” test as “causal”, ignoring the lack of clinical evidence, biological plausibility, or the absence of interventions that are even remotely effective. In addition, most women suffering either repeated implantation failure or multiple miscarriages ‘explained’ or not, will eventually achieve a successful pregnancy irrespective of treatment (11-13). For example, several randomized-controlled trials on RPL, defined here as three consecutive pregnancy losses, reported life-births rate of 65% or more in the placebo group (14,15). The situation is further complicated by the now confirmed observation that preclinical pregnancy loss may exceed 50% (16-18). Indeed, human fecundity rarely exceeds 35% and may be decreasing due to deteriorating semen quality (19).
It is for this reason that male factors may well be important in causing embryonic loss, but, mixed with all other variables, their effect may be masked. Factors that may influence the number, motility and morphological features of spermatozoa include occupation, exposure to environmental toxins and smoking habits (20-22).
In the minds of many practitioners, intracytoplasmic sperm injection (ICSI) (23) renders detailed sperm analysis unnecessary. Yet, already in the nineties it was documented that embryo viability may be compromised when fertilization is achieved using an abnormal spermatozoon (24). Indeed, in 1997, Hamamah et al. (25) demonstrated a relationship between poor semen quality and poor embryonic development; and reported an increased incidence of abnormal sperm morphology in couples suffering from RPL. It has been shown that successful fertilization in humans requires centrosome restoration and microtubule-mediated motility and that the sperm introduces the centrosome (24-26).
Thus, while ICSI has improved dramatically the management of male factor subfertility, careful sperm analysis remains potentially a valid test when investigating persistent reproductive failure. The situation, however, is complex. In 2000, Wennerholm et al. (27) evaluated the outcome of 1,293 clinical pregnancies according to sperm quality. Their results indicate that sperm origin or quality is not associated with preterm birth, although there appears to be a correlation with multiple births. Also, perinatal mortality rates did not differ according to sperm quality. This led to the conclusion that obstetric outcome following ICSI is similar to that of conventional IVF and not influenced by sperm origin or quality. Carrell et al. (28) reported significantly higher aneuploidy rates in the sperm of partners of women with RPL when compared to general or fertile populations (P<0.005). They concluded that, at least in some cases of RPL, partners have a significant increase of abnormal sperm morphology, chromosome aneuploidy or apoptosis. This finding appeared to be supported by a study using in situ hybridization (29).
More recently, an important study by Lin et al. (30) attempted to correlate sperm chromatin structure assay (SCSA) parameters, DNA fragmentation index (DFI) and high DNA stainability (HDS) with the outcomes following IVF and ICSI. No significant differences were found in fertilization rates, the number of good quality embryos and the likelihood of pregnancy between high, moderate, and low DFI or HDS groups. At the same time, men with HDS >15% had significantly increased miscarriage rates following IVF. A similar but non-significant trend was observed in the high DFI group.
Given the conflicting findings, Gil-Villa et al. (31) further investigated sperm characteristics in order to determine the relationship with RPL using standard sperm parameters, lipid peroxidation of sperm plasma membranes, antioxidant capacity of seminal plasma, sperm chromatin integrity and DNA fragmentation tests. Following a full comparison, the investigators reported that RPL is associated with a higher incidence of teratozoospermia. In yet another study, sperm DNA fragmentation in seminal ejaculates in men whose partners had a history of RPL was compared to that of men with proven fertility (32). A significant difference was observed in sperm motility, but not in other parameters. However, the number of sperm with fragmented DNA was significantly increased in the group of men whose partners had RPL. The authors concluded that a higher incidence of DNA damage and poor motility can explain, at least in part, pregnancy loss in their partners.
Like sunshine after rain, these results were contradicted by a prospective study where the rate of DNA damage was measured in fresh and processed ejaculated sperm. Starting from the observation that rates of aneuploidy and the index of DNA fragmentation are higher in poor-quality sperm samples, Bronet et al. (33) assessed the relationship between sperm DNA fragmentation and aneuploidy rates in spermatozoa and embryos in couples suffering from RPL. They found no correlation between the extent of DNA fragmentation and the rate of aneuploidy in embryos or sperm, implying that sperm DNA fragmentation does not correlate with embryonic aneuploidies. Whether or not different methods to assess DNA fragmentation would have produced different results remains an open question.
We have tried to summarize this conflicting evidence in Table 1.
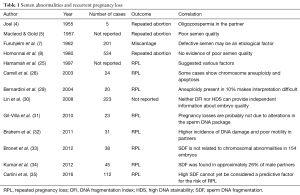
Full table
In the absence of unequivocal evidence, the question arises whether these tests are clinically useful in RPL, whether “explained” or not. Kumar et al. (34) applied receiver operating curve (ROC) analysis to semen samples from 45 patients whose spouses had “idiopathic” RPL and 20 normally fertile controls. As DNA damage was higher in RPL couples, the authors concluded that sperm DFI is useful in the management of affected couples. This conclusion appears to be supported by the findings of Carlini et al. (35), who also reported a correlation between increased sperm DNA fragmentation in men from couples reporting two or more spontaneous abortions and impaired reproductive capacity in terms of both rates of fertilization and of pregnancies with viable offspring. Clearly, the information gleaned from small studies needs to be interpreted with care, and large, well-designed prospective studies are urgently needed.
Based on current evidence, the American Society for Reproductive Medicine (ASRM) Selective Practice Guidelines (36) emphasize that the relationship between sperm DFI and miscarriage following IVF or ICSI remains unproven. Therefore, there is no sound clinical basis as yet to recommend inclusion of sperm DNA integrity among routinely mandated tests.
One final technical comment relates to the fact that methods to evaluate chromatin integrity can only measure the percentage of cells with fragmented DNA and are based on the idea that the greater the fragmentation rate, the greater the chance that the sperm population is pathological (37). The problem is that no definite threshold of DNA damage beyond which a seminal sample can be considered pathological has been agreed and different studies provided different information, since stratifying results on the basis of the methodology applied gives different results (38). The majority of investigations reporting a significant impact following either IVF or ICSI, of the level of sperm DNA fragmentation on blastocyst and embryo development and on miscarriage rate, utilized the TUNEL technique. On the contrary, using SCSA variable results have been obtained (39).
In conclusion, presently available sperm chromatin integrity tests represent a useful research tool allowing the study of chromatin structure, as well as of the origin and mechanisms of DNA damage. Implementation in clinical practice, however, is not yet supported because of a lack of robust evidence. Clearly, there is an urgent need for the standardization of the methods and for additional clinical studies on the impact of SDF on ART outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Cissen M, Wely MV, Scholten I, et al. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0165125. [Crossref] [PubMed]
- Kirkman-Brown JC, De Jonge C. Sperm DNA fragmentation in miscarriage - a promising diagnostic, or a test too far? Reprod Biomed Online 2017;34:3-4. [Crossref] [PubMed]
- Joel CA. The role of spermatozoa in habitual abortion. Fertil Steril 1955;6:459-64. [Crossref] [PubMed]
- Macleod J, Gold RZ. The male factor in fertility and infertility. IX. Semen quality in relation to accidents of pregnancy. Fertil Steril 1957;8:36-49. [Crossref] [PubMed]
- Furuhjelm M, Jonson B, Lagergren CG, et al. The quality of the human semen in relation to perinatal mortality. Acta Obstet Gynecol Scand 1960;39:499-505. [Crossref] [PubMed]
- Furuhjelm M, Jonson B, Lagergren CG. The quality of human semen in spontaneous abortion. Int J Fertil 1962;7:17-21. [PubMed]
- Homonnai ZT, Paz GF, Weiss JN, et al. Relation between semen quality and fate of pregnancy: retrospective study on 534 pregnancies. Int J Androl 1980;3:574-84. [Crossref] [PubMed]
- Larsen EC, Christiansen OB, Kolte AM, et al. New insights into mechanisms behind miscarriage. BMC Med 2013;11:154. [Crossref] [PubMed]
- Vaiman D. Genetic regulation of recurrent spontaneous abortion in humans. Biomed J 2015;38:11-24. [Crossref] [PubMed]
- Saravelos SH, Regan L. Unexplained recurrent pregnancy loss. Obstet Gynecol Clin North Am 2014;41:157-66. [Crossref] [PubMed]
- Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod 1999;14:2868-71. [Crossref] [PubMed]
- Ogasawara M, Aoki K, Okada S, et al. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril 2000;73:300-4. [Crossref] [PubMed]
- Coomarasamy A, Williams H, Truchanowicz E, et al. A Randomized Trial of Progesterone in Women with Recurrent Miscarriages. N Engl J Med 2015;373:2141-8. [Crossref] [PubMed]
- Pasquier E, de Saint Martin L, Bohec C, et al. Enoxaparin for prevention of unexplained recurrent miscarriage: a multicenter randomized double-blind placebo-controlled trial. Blood 2015;125:2200-5. [Crossref] [PubMed]
- Zinaman MJ, Clegg ED, Brown CC, et al. Estimates of human fertility and pregnancy loss. Fertil Steril 1996;65:503-9. [Crossref] [PubMed]
- Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189-94. [Crossref] [PubMed]
- Wang X, Chen C, Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril 2003;79:577-84. [Crossref] [PubMed]
- Benagiano G, Farris M, Grudzinskas G. Fate of fertilized human oocytes. Reprod Biomed Online 2010;21:732-41. [Crossref] [PubMed]
- Edmonds DK, Lindsay KS, Miller JF, et al. Early embryonic mortality in women. Fertil Steril 1982;38:447-53. [Crossref] [PubMed]
- Parazzini F, Bocciolone L, Fedele L, et al. Risk factors for spontaneous abortion. Int J Epidemiol 1991;20:157-61. [Crossref] [PubMed]
- Bulletti C, Flamigni C, Giacomucci E. Reproductive failure due to spontaneous abortion and recurrent miscarriage. Hum Reprod Update 1996;2:118-36. [Crossref] [PubMed]
- Palermo G, Joris H, Devroey P, et al. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17-8. [Crossref] [PubMed]
- Simerly C, Wu GJ, Zoran S, et al. The paternal inheritance of the centrosome, the cell's microtubule-organizing center, in humans, and the implications for infertility. Nat Med 1995;1:47-52. [Crossref] [PubMed]
- Hamamah S, Fignon A, Lansac J. The effect of male factors in repeated spontaneous abortion: lesson from in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod Update 1997;3:393-400. [Crossref] [PubMed]
- Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol 1994;165:299-335. [Crossref] [PubMed]
- Wennerholm UB, Bergh C, Hamberger L, et al. Obstetric outcome of pregnancies following ICSI, classified according to sperm origin and quality. Hum Reprod 2000;15:1189-94. [Crossref] [PubMed]
- Carrell DT, Wilcox AL, Lowy L, et al. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstet Gynecol 2003;101:1229-35. [PubMed]
- Bernardini LM, Costa M, Bottazzi C, et al. Sperm aneuploidy and recurrent pregnancy loss. Reprod Biomed Online 2004;9:312-20. [Crossref] [PubMed]
- Lin MH, Kuo-Kuang Lee R, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril 2008;90:352-9. [Crossref] [PubMed]
- Gil-Villa AM, Cardona-Maya W, Agarwal A, et al. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril 2010;94:1465-72. [Crossref] [PubMed]
- Brahem S, Mehdi M, Landolsi H, et al. Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology 2011;78:792-6. [Crossref] [PubMed]
- Bronet F, Martínez E, Gaytán M, et al. Sperm DNA fragmentation index does not correlate with the sperm or embryo aneuploidy rate in recurrent miscarriage or implantation failure patients. Hum Reprod 2012;27:1922-9. [Crossref] [PubMed]
- Kumar K, Deka D, Singh A, et al. Predictive value of DNA integrity analysis in idiopathic recurrent pregnancy loss following spontaneous conception. J Assist Reprod Genet 2012;29:861-7. [Crossref] [PubMed]
- Carlini T, Paoli D, Pelloni M, et al. Sperm DNA fragmentation in Italian couples with recurrent pregnancy loss. Reprod Biomed Online 2017;34:58-65. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril 2013;99:673-7. [Crossref] [PubMed]
- Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 2009;30:219-29. [Crossref] [PubMed]
- Simon L, Brunborg G, Stevenson M, et al. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod 2010;25:1594-608. [Crossref] [PubMed]
- Tamburrino L, Marchiani S, Montoya M, et al. Mechanisms and clinical correlates of sperm DNA damage. Asian J Androl 2012;14:24-31. [Crossref] [PubMed]


