Use of botulinum toxin for voiding dysfunction
Introduction
The use of botulinum toxin A (BoNT-A) for the treatment of lower urinary tract conditions has rapidly expanded over the last two decades. At present, BoNT-A has become a well-established therapy in the management of neurogenic detrusor overactivity (NDO) and idiopathic overactive bladder (OAB). Although these are the only licensed indications within the urinary tract, there are a wide range of off-license indications including bladder pain syndrome (BPS), detrusor sphincter dyssynergia (DSD) and benign prostatic hyperplasia (BPH).
Botulinum toxin (BoNT) was first isolated and purified as crystalline product in 1946 (1) and was initially used to treat ocular strabismus in 1977 (2). Subsequently, the treatment spread to a broad range of conditions associated with muscular hyperactivity, glandular hypersecretion and inflammation (3). The initial use within the urinary tract was described in 1988 by Dykstra et al. who injected BoNT-A into the external urethral sphincter to treat DSD in patients with spinal cord injury (SCI) (4). This remained the single application until Schurch et al. published a landmark paper on intravesical BoNT-A injections for NDO (5). This was followed by a rapid expansion in the application of BoNT-A across idiopathic detrusor overactivity (IDO) (6,7), BPS (8) and BPH (9).
As the range of applications for BoNT-A continues to expand, this article reviews the current evidence for the most common indications for BoNT-A in the lower urinary tract. The review focuses on BoNT-A injections in adults with NDO, OAB, PBS/IC or BPH. The most recent published literature is critically evaluated and we summarize the mechanism of action, injection technique, efficacy and adverse events (AEs) for each indication.
Types of BoNT
BoNT is a potent neurotoxin synthesized by the gram positive, aerobic spore-forming bacterium Clostridium botulinum. BoNT serotypes are synthesized as an inactive single-chain polypeptide which is activated when cleaved into a 50 kDa light chain and a 100 kDa heavy chain. The heavy chain is responsible for transport of the light chain into the neuronal cytosol and the main pharmacological action is provided by the light chain which acts on the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex inhibiting the release of neurotransmitters into the synaptic cleft.
There are seven immunologically distinct serotypes from type A to type G which have been isolated. The most commonly used serotype within the lower urinary tract is BoNT-A. It is available in different commercial forms and the two most common preparations are onabotulinumtoxin A (onaBoNT-A) (Botox®; Allergan, Ltd., Irvine, USA) and abobotulinumtoxinA (aboBoNT-A) (Dysport; Ipsen Ltd., Slough, UK). Although these preparations have similarities, their manufacturing processes have different isolation, extraction, purification and stabilization processes (10). This results in products with different molecular characteristic and dosing requirements and they should not be considered as generic equivalents.
Detrusor overactivity (DO)
NDO and OAB remain the only approved indications for BoNT-A within the urinary tract. onaBoNT-A has received regulatory approval from the U.S. Food and Drug Administration (FDA) (11) and the UK Medicine and Healthcare Products Regulatory Agency (MHRA) (12). It is recommended by the majority of international bodies and guidelines as a second-line treatment for NDO or OAB in patients who have symptoms refractory to antimuscarinics or β3 adrenoceptor agonists (13,14).
Mechanism of action
BoNT-A appears to have a dual mechanism of action on both the motor and sensory pathways responsible for DO (15). The original research into BoNT-A was in skeletal muscle which suggested that the mechanism of action was solely due to inhibition of acetylcholine release from presynaptic efferent nerves (16). This occurs when BoNT-A enters the presynaptic neuron by binding to the synaptic vesicle 2 (SV2) receptor protein. BoNT-A enters the nerve by endocytosis and the light chain and heavy chain separate in the endosomal vesicle. The light chain is translocated into the cytosol where it cleaves the SNAP-25 protein which is an essential component for fusion of vesicles containing acetylcholine with the neuronal cell membrane (17,18). Blocking the release of acetylcholine inhibits parasympathetic signalling to the bladder, reducing involuntary detrusor contractions.
In addition to inhibiting detrusor activity, it was noted that patients described improvements in sensory symptoms which highlighted that BoNT-A may also modulate sensory functions in an unrelated mechanism to inhibition of acetylcholine release. Animal studies have shown that BoNT-A inhibits the release of a range of neurotransmitters from the urothelium including CGRP (10), substance P (11) and ATP (12). This is combined with decreasing expression of sensory receptors such as vanilloid (TRPV1) and purinergic (P2X3) receptors further modulate sensory function (13). Beyond the peripheral sensory effects, animal studies have found that BoNT-A may reach the CNS by retrograde axonal transport and have central antinociceptive activity (14). Both motor and sensory effects are reversible but regeneration of sensory receptors appears to take longer and it is the sensory effects which determine the duration of action of BoNT-A (19).
Techniques for injection and dosing
There remains no standardized injection technique and significant variability exists between centres. Factors which vary include use of rigid or flexible cystoscopy, general or local anaesthetic, size of injection needle and optimal injection site. The technique was originally described in NDO patients using a collagen needle with a rigid cystoscope (5). However, many centres have adopted a minimally invasive, local anaesthetic approach known as the “Dasgupta technique” (20). The injections are performed using a flexible cystoscope and an ultra-fine 4 mm needle. Local anaesthetic is administered prior to the procedure with 2% intra-urethral lidocaine gel. The technique avoids risk of a general anaesthetic and has significant cost advantages (21). Procedure time is approximately 15–20 mins and it is well tolerated with low patient reported pain scores (22).
The majority of centres use an injection protocol which includes 20–30 injections sparing the trigone. Traditionally the trigone has been spared based on a theoretical risk of inducing vesicoureteral reflux (VUR). However, there remains discussion regarding the optimal injection sites and multiple studies have challenged the VUR theory using video urodynamics to show that trigonal injections do not induce VUR in both NDO (23) and OAB (24) patients. It has been suggested that protocols which include the trigone may have additional sensory benefits as the trigone has a high density of nociceptive bladder afferents (25). An randomized controlled trial (RCT) which compared trigone-sparing and trigone-including injection in found that the trigone injections improved overall symptom scores and urgency subscale scores in IDO patients (26). However, a recent meta-analysis by Davis et al. did not find any difference in short term efficacy between trigonal and extratrigonal injections (27).
Dosing
The licensed dose in NDO has been set at onaBoNT-A 200 U based on several phase 3 RCTs. These studies found no difference in efficacy outcomes between 200 and 300 U but the higher dose was associated with a significant risk of clean intermittent self-catheterization (CISC) (28,29). Similarly in OAB, the licensed dose is onaBoNT-A 100 U following several large dose ranging RCTs (30-32). These doses were seen as the optimal risk-benefit ratio but were lower than had been previously utilized in the early studies.
Clinical efficacy in NDO
The initial description of BoNT-A as a treatment for NDO was a pilot study by Schurch et al. who treated 21 patients with SCI using BoNT-A 200 or 300 U sparing the trigone (5). All patients had failed maximum anticholinergic treatment and were CISC dependent. At 6 weeks, 17 out of 19 patients were completely continent and ten patients had decreased anticholinergic requirements. There were also baseline improvements in urodynamic parameters including mean maximum cytometric capacity (MCC) and maximum detrusor pressure (PDetmax).
These promising initial results triggered several small placebo controlled, randomized studies evaluating the use of BoNT-A in NDO which are summarized in Table 1 (33-35). The first RCT randomized 59 patients with SCI and MS to two doses of BoNT-A (200 and 300 U) and placebo saline injections (33). The primary end points were incontinence episodes which were reduced in the 200 U group at 24 weeks and in the 300 U group at 2 and 6 weeks. There was a significant improvement in quality of life (QoL) scores in both treatment groups compared to placebo. No difference was found in outcomes between the two doses which were expected as the study was not powered to detect differences between groups.
The highest level evidence is dominated by the results of two phase III, double-blind, placebo-controlled trials (28,29). These two pivotal phase III studies will be discussed together as their data has been pooled in subsequent post hoc analysis (36,37). It was following their publication that BoNT-A was approved for treatment of urinary incontinence secondary. The pooled data includes 691 patients with either MS (n=381) or SCI (n=310) who had >14 urgency incontinence (UI) episodes per week and had symptoms refractory to anticholinergics for >1 month duration. The cohort was randomized to onaBoNT-A 200 U, onaBoNT-A 300 U or placebo and the primary end point was change from baseline in mean UI episodes at week 6. Secondary outcomes included urodynamics parameters and incontinence quality of life (I-QoL) scores. At 6 weeks, there was a statistically significant improvement in UI episodes per week in both treatment arms compared to placebo regardless of NDO aetiology. In addition, a higher percentage of patients were dry at 6 weeks compared to placebo (MS: 41.5%; SCI: 30.9%). A dose comparison showed no additional treatment efficacy from a higher 300 U dose. In terms of secondary urodynamic end points there were significant improvements in MCC, number of involuntary detrusor contraction and Pdetmax during first IDC. The detailed outcomes on UI episodes, urodynamic and QoL scores are shown in Table 1.
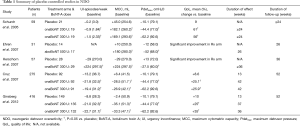
Full table
The long-term efficacy outcomes of BoNT-A injections have been evaluated in a 4-year, prospective, multicentre extension study (38). This study included 396 patients from the original phase III studies who received up to six repeat injections. It showed that there were sustained improvements in UI episodes per week and I-QoL scores across treatment cycles. The median treatment duration was 9 months and no new safety concerns were identified.
An important issue to consider is that these phase III studies only included patients with multiple sclerosis (MS) and SCI. There is considerable heterogeneity in aetiology of NDO and we must rely on non-randomized studies to confirm that BoNT-A injections are effective in other NDO aetiologies such as Parkinson’s, multiple system atrophy, chronic cerebrovascular accidents and spinal cord lesions (39,40). In terms of BoNT-A formulations, the majority of studies have investigated the use of onaBoNT-A, but there is evidence for the clinical efficacy on aboBoNT-A. A placebo controlled RCT by Ehren et al. found a reduction in UI episodes, urodynamic parameters and QoL scores using aboBonT-A 500 U injections (34).
Clinical efficacy in OAB
The use of BoNT-A injections as a treatment for OAB or IDO has grown rapidly since first described in 2001 (6,7). During this time, the definition of OAB has moved to a symptom-based clinical diagnosis characterized by urinary urgency usually accompanied by frequency and nocturia with or without UI in the absence of urinary tract infection (UTI) (41). Sahai et al. reported the first randomized, double-blind, placebo-controlled trial which evaluated the efficacy and safety of onoBoNT-A 200 U in patients with IDO (42). The primary end point was change in MCC which was found to have significantly increased at 3 months. There were also improvements in QoL scores, OAB symptoms and urodynamic parameters in favour of onaBoNT-A compared to placebo. The detailed results are summarized in Table 2. Following this study, two other small RCT were published showing similar results in female patients (43) and across different doses of onaBoNT-A (200 and 300 U) (44). A meta-analysis of these early RCTs by Anger et al. (45) confirmed the efficacy of BoNT-A at improving OAB symptoms and QoL scores.
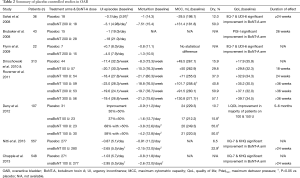
Full table
Subsequently, there have been several dose-escalation placebo-controlled studies to establish the optimal BoNT-A dose (30-32). The largest was a phase II, multi-centre, double-blind RCT by Dmochowski et al. which randomized 313 patients to different doses of onaBoN-T (50, 100, 150, 200 and 300 U) and placebo. The study included both patients with IDO and bladder oversensitivity, defined as OAB symptoms without demonstrable DO. The authors concluded that there was a significant improvement in OAB symptoms and QoL scores at all doses of 100 U and above. The presence of DO was not a predictive factor for outcome. A non-parametric analysis found that the reduction in urgency urinary incontinence (UUI) was dose dependent. The lower dose of 50 U was not as effective as higher doses. There was minimal additional benefit at doses above 150 U and these were associated with significantly higher rates of raised for post-void residual (PVR) and need for CISC. Similar findings were identified by a subsequent smaller dose ranging study by Denys et al. which compared 50, 100, 150 U onaBoNT-A with placebo and found that the two highest doses were most efficacious but 100 U had a lower risk of raised PVR and need for CISC (32). Based on these dose-ranging studies, the dose was set at 100 U onaBoNT-A as the optimal balance between treatment efficacy and AEs.
The final step to licensing was the completion of two large, placebo-controlled phase III studies (46,47) which have been pooled by Sievert et al. giving a sample size of 1,105 patients (48). The enrolled patients had and ≥3 UI episodes per three days, ≥8 micturitions per day and were randomized to 100 U onaBoNT-A (n=557) and placebo (n=548). The study found a significant decline in urinary urgency incontinence (UUI) episodes per day in the treatment arm compared to placebo (−2.80 vs. −0.95 episodes/day; P<0.001). At week 12, full continence was achieved in 27.1% of onaBoNT-A group vs. 8.4% in the placebo group (P<0.001). The median time to request re-treatment was 24 weeks for onaBoNT-A compared to 12 weeks in placebo group.
Previous studies had suggested that BoNT-A was more likely to be effective in patients who were unable to tolerate anticholinergics rather than poor medication efficacy (49). However, a sub-analysis in the phase III studies showed that efficacy of BoNT-A was not affected by the reason for discontinuation of medical therapy or the number of prior anticholinergics.
The long-term efficacy of onaBoNT-A has been recently evaluated in a prospective, multicenter, 3.5-year extension study. This included 839 patients who had been enrolled in the original phase III studies and were invited to continue long-term follow-up (50). The cohort received up to six repeat injections and there was a consistent absolute reduction of UI episodes, ranging from −3.1 to −3.8, across each treatment cycle. The median duration of effect was 7.6 months and no new AEs were identified.
AEs
The most common AEs from BoNT-A injections are UTI and voiding dysfunction requiring CISC. The pooled analysis of phase 3 trials reported uncomplicated UTI rates of 53.8% in NDO (37) and 25.5% in OAB (48). The definition of UTI is variable between studies with the OAB trials required a positive urine culture while NDO trials based the diagnosis on clinical assessment. Urinary retention and need for CISC is the next most common AE and these are known to be dose dependent. In NDO, for patients who were not CISC dependent at baseline, the risk of requiring de novo CISC was 30.8% and 44.0% in onaBoNT-A 200 and 300 U groups respectively (37). In OAB, the risk of retention was 5.8% for onaBoNT-A compared to 0.4% with placebo (48). The risks of systemic side effects including generalised muscle weakness, dysphagia and respiratory depression are very rare.
The long-term extension studies did not identify any new AEs following repeat injections. There had been early concerns that repeat injections could cause fibrosis, reduced bladder compliance and worsen of overactive symptoms but this has not been demonstrated in clinical studies (51).
Bladder pain syndrome (BPS)
Interstitial cystitis (IC) or BPS is a chronic condition characterized by debilitating bladder pain of unknown aetiology. The recent EAU guidelines have recommended a new nomenclature in which BPS is the singular term used and combinations such as PBS/IC are no longer recommended (52). The guidelines defines BPS as a recurrent pain, perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 months duration and not associated with obvious local pathology (52). There is no standardized treatment regimen but there are a multitude of therapies which are often unsuccessful at completely eradicating the syndrome. The current treatments are usually performed in a stepwise approach including physiotherapy, oral medications, endourological procedures (intravesical installations, hydrodistention, laser fulguration) neuromodulation and cystectomy. Although BoNT-A injections remain unlicensed for BPS, the recent AUA guidelines have recommended them as a fourth line treatment in patients who failed conventional options (53).
Mechanism of action
The aetiology and pathogenesis of BPS are still not clearly understood and this contributes to the challenge of identifying effective treatments (54). Various aetiological factors have been suggested such as subclinical or chronic infection, autoimmune mechanisms, allergic processes and exposure to toxins (55). The predominate histological findings in BPS are denudation of the glycosaminoglycan urothelial surface layer, mucosal ulceration, neuronal upregulation and inflammatory cell activation, which suggests an underlying inflammatory process in the disease (56). BoNT-A has been hypothesized as having a multifactorial action by improving urothelial dysfunction, reducing inflammatory cell activation and modulating sensory function (57). In addition to the sensory effects, animal studies have shown that BoTN-A inhibits sensory neuropeptide release in inflammatory rats suggesting a potential clinical benefit in reducing neurogenic inflammation (58)
Techniques for injection and dosing
The general injection technique is similar to DO and the majority of studies use a dose of 100 or 200 U onaBoNT. A RCT comparing these doses found that rates of AEs were higher in the 200 U group with no significant difference in efficacy (59). There is no consensus on the optimum injections site in BPS (60). The discussion regarding injections in the trigone has been of increased importance in BPS given the high density of nociceptive bladder afferents in the trigone (25). The majority of BPS studies have shown that BoNT-A injections can produce significant improvements in pain, symptom scores and urodynamic parameters (8,25,61,62). However, trigone sparing injection protocols have not produced consistent clinical improvements (59) which suggests that trigone-including protocols may be important. However, there is a need for a specific comparison study to formally evaluate this.
Clinical efficacy
There have been three published placebo-controlled RCTs providing level 1 evidence for BoNT-A injections in BPS (59,63,64). These studies have significant heterogeneity in terms of inclusion criteria, definition of BPS, efficacy outcomes, BoTN-A dose and site of injection. The results are summarized in Table 2. The first RCT did not show any improvement in pain scores (63) although this was not unexpected as the study used a novel periurethral injection technique with the aim of inhibiting urethral, visceral and somatic afferent fibres. A subsequent RCT using a standard suburothelial injection technique compared the efficacy of hydrodistention alone with two doses of onaBoNT-A injections (100 and 200 U) followed by hydrodistention. In this study pain scores, functional bladder capacity and cystometric bladder capacity were significantly improved in the onaBoNT-A groups compared to the control group at 3 months follow-up (P=0.02).
These results were confirmed in a recent multi-centre, randomized, double-blind, placebo-controlled trial which recruited 60 patients randomized to BoTN-A 100 U or normal saline injections. All received twenty suburothelial injections sparing the trigone after hydrodistention under general anaesthetic. The primary outcome measure was reduction in VAS pain score which was found to be significantly improved in the BoNT-A group compared to the control (−2.62 vs. −0.9, P=0.021) (64). There was no significant difference demonstrated in urodynamic parameters apart from maximum bladder capacity which was higher in the BoNT-A group.
Given that BPS encompasses a heterogeneous spectrum of disorders, it is hypothesized that it may be more effective in certain sub-groups. Lee et al. compared the efficacy of BoNT-A in the presence or absence of Hunner’s ulcers (65) and found no benefit in ulcerative IC across VAS scores, O’Leary-Sant scores, frequency and bladder capacity. The study concluded that BoNT-A may only be effective in non-ulcerative IC with approximately 50% of patients reporting a clinical benefit from treatment.
AEs
The AEs are similar to those reported in NDO and OAB studies. The risk has also been shown to be dose related in an RCT by Kuo et al. which found a higher incidence of dysuria and urinary retention with onaBonT-A 200 U compared to onaBoNT-A 100 U (59).
Detrusor sphincter dyssnergia (DSD)
The use of BoNT-A injections has been applied to target organs outside the bladder including the external urethral sphincter. DSD is characterized by involuntary sporadic contractions of the urethral sphincter during a detrusor contraction, due to a CNS lesion between the sacral spinal cord and pontine micturition centre (66). It can lead to incomplete bladder emptying, high pressure retention, VUR and renal impairment. DSD is typically seen in patients with supra-sacral spinal lesions (SCI), MS, myelomeningocele and acute transverse myelitis (67). The first application of BoNT-A within the lower urinary tract was by Dykstra et al. who injected BoNT into the external urethral sphincter of patients with SCI to treat (4). The effects can be treated by CISC but this may not be physically possible for certain patient groups such as MS and quadriplegic patients. Therefore, BoNT-A injections for DSD have been primarily used in patients who were unable to perform CISC as an alternative to surgical sphincterotomy.
Mechanism of action
The effects of BoNT-A on striated muscle has been extensively studied in conditions associated with dystonic and spastic muscular hyperactivity (68). By blocking the presynamic release of acetylcholine, there is chemo-denervation of the target muscle. The aim is to produce a “chemical sphincterotomy” where there is sufficient reduction in external sphincter tone to improve voiding dysfunction. The electromyography after BoNT-A injections in Dykstra’s et al.’s original report confirms a decrease in maximum urethral pressure (MUP) by an average of 27 cmH2O (4).
Techniques for injection and dosing
A range of injection techniques into the external urethral sphincter have been described including a transurethral, paraurethral and transperineal approach with or without electromyography guidance (69). The efficacy appears to be equivalent across each technique and the particular approach will be determined by operator experience and equipment availability (70). The transurethral approach has been frequently described in men and involves BoNT-A injected directly into the external urethral sphincter under rigid or flexible cystoscopic guidance (67). The injections are placed into the sphincter at four to eight sites, typically in 3, 6, 9 and 12 o’clock positions, and the needle needs to be inserted at a depth of approximately 1 cm, which is deeper than in urethral bulking agents, to avoid the suburothelial space and ensure the drug reaches the sphincter (71). This approach may require a general or spinal anaesthetic but has been described under local anaesthetic. A periurethral approach can be performed in women which involves inserting a needle transcutaneously, adjacent to the urethra, at a depth of approximately 1.5 cm to reach the external sphincter (72).
Transperineal techniques have been well described combined with electromyography (EMG) (73) or transrectal ultrasound (70) for localisation of the external urethral sphincter. EMG-guided methods are technically challenging and it is difficult to exclude interference from the surrounding perineal muscles (74). Nevertheless, MRI studies have shown that an experienced operator using EMG can accurately target the external urethral sphincter (74). An alternative is transrectal ultrasound-guided transperineal injection which more clinicians are gaining experience of as an investigation in prostatic disease. A transrectal three-dimensional multiplanar transducer probe can be used to identify the hypoechoic external urethral sphincter located just distal to the prostatic apex (70).
The dose of BoNT-A injected into the external urethral sphincter ranges from 50 to 200 U of onaBoNT-A (75,76) or 150 U of aboBoTN-A (77). The drug is usually reconstituted in 2 to 4 mL of normal saline.
Clinical efficacy
Since Dykstra’s initial report, there have been only three small RCTs completed in DSD which are summarized in Table 2. A small RCT was conducted by Dykstra et al. 1990 including five patients with SCI randomized to BoNT-A 140 U and normal saline (78). At day 21, there was a decrease in MUP, PVR and maximum bladder pressure in the treatment arm. The results from the placebo group are not reported so no comparisons can be drawn regarding any placebo effect. Another small active-comparator RCT randomized 13 patients to BoNT-A 100 U and lidocaine 0.5%. This trial did find a significant decrease in PVR in the treatment group compared to lidocaine (−159.4 vs. 49.8 mL) although the mean PVR remained elevated at above 100 mL (79). A larger multi-centre RCT by Gallien et al. randomized 86 patients with MS to a single transperineal injection of BoNT-A 100 U or saline as placebo (80). The primary outcome measure was PVR, measured using catheterisation, and secondary outcomes included voiding and urodynamic variables. There was no significant difference in PVR between the two groups (P=0.45) although some differences were found in urodynamic parameters.
The results from the RCT conflict with subsequent small heterogeneous observational studies which demonstrate that PVR is significantly reduced following BoNT-A injections. Other observational studies have found improvements in QMax, maximum urethral closure pressure, frequency of voiding and QoL. A meta-analysis of SCI patients also found that there was a mean PVR decrease from 251.8 to 153.0 mL for up to 6 months (81).
Nevertheless, a recent Cochrane review which reviewed all of this literature concluded that the current evidence for BoNT-A in DSD is of limited quality due to the small number of participants in level 1 trials and the risk of bias from observational studies (67). The review noted that a surgical sphincterotomy may be the superior treatment as it provides greater efficacy and longer duration effect.
AEs
BoNT-A injections were generally well tolerated with minimal adverse side effects. Dykstra et al. 1990 injected 140 U BoNT-A followed by 240 U at subsequent sessions and reported three episodes of transient muscle weakness. This was thought to be due to a short inter-injection interval of 1 week (78) and the authors have addressed this by waiting a minimum of 2 weeks between injections (67). Observational studies have reported the exacerbation of stress urinary incontinence following injection due to the denervation of the external urethral sphincter. This has been shown to be a major cause of patient dissatisfaction following the treatment (82). Therefore it is important to appropriately counsel them about the risk of stress incontinence before commencing this off-licence treatment.
BPH
Since first reported in 2003, there has been significant interest in BoNT-A prostatic injections as an alternative treatment for BPH (9). Current medical treatment for BPH is associated with autonomic and sexual side effects and a proportion of patients’ progress despite combination therapy. Surgical techniques are associated with long-term morbidity including retrograde ejaculation and erectile dysfunction and a less invasive alternative is highly desirable.
Mechanism of action
The exact mechanisms of action remains under discussion but a dual mechanism has been proposed in which BoNT-A injections act on both the static and dynamic components of BPH (83). The prostate receives autonomic innervation from cholinergic fibres supplying predominately the epithelium and noradrenergic fibres supplying the prostatic stroma (84). Muscarinic receptors are expressed in abundance on the prostatic epithelium (85,86) and it is postulated that these play a key role in prostatic growth in combination with testosterone (87). BoNT-A injections inhibits the influence of acetylcholine on these receptors resulting in disruption of the excessive growth (static) component of BPH. Atrophy of the gland and subsequent reduction in prostate volume should improve the obstructive symptoms associated with BPH.
The regulation of the smooth muscle (dynamic) component was originally thought to be due to an inhibition of the effects of noradrenaline on the prostatic stroma (84). More recent studies have suggested that BoNT-A effects on stromal smooth muscle is due to a combination of down regulating α1A-adrenoceptors, vacuoles forming in stromal smooth muscle cells and inhibition of norepinephrine release from sympathetic fibres (83).
Techniques for injection and dosing
There is no standardized injection technique but the majority of studies describe a similar technique of two injections of equal volume (2 mL) into the transition zone of each lateral prostatic lobe (9,88-93). Kuo et al. (94) described an alternative technique of ten injection sites including the middle lobe while Marberger el al. (95) describes a three site technique which includes the cranial, middle and caudal parts of each lateral lobe.
Most studies describe BoNT-A injections using a transperineal approach (9,88-92,94,96) (Table 3). It might be expected that the transrectal route would be associated with a high risk of infection due to bacterial contamination from rectal flora. This has been demonstrated in prostatic biopsy where the transperineal approach has an infection rate approaching zero (97) compared to up to 6.3% after transrectal biopsy (98). The transrectal and transperineal approach were compared in a large randomized control trial by Marberger et al. although this was not part of the trial protocol and the comparison was only possible due to an amendment to the trial protocol. There was no significant difference in clinical efficacy based on route of administration. However, a high rate of prostatitis was reported in the transrectal group compared to the transperineal group.
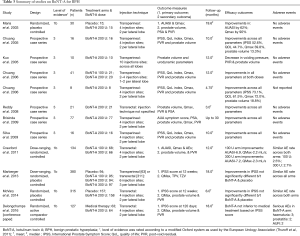
Full table
The most common dosing is with onaBoNT-A 200 U distributed equally into each lateral lobe. However, the two phase II, dose escalation, RCTs concluded that there was no significant difference in efficacy or AEs between 100–300 U (95,99). Crawford et al. concludes that 100 U dose may be preferable based on the similar efficacy and adverse effects with reduced cost. An alternative approach is to stratify the dose based on prostate size. Chuang et al. used 100 U for prostate volumes <30 mL and 200 U for prostate volumes >30 mL (96).
Clinical evidence
The potential application of BoNT-A for BPH in humans was first investigated by Maria et al. in 2003 (9). This placebo-controlled RCT randomized 30 patients with symptomatic BPH to placebo or BoNT-A 200 U. The primary outcome measures were improvement in QMax and subjective BPH symptomatic improvement based on the AUA symptom improvement score. At 2 months follow-up, there had been a subjective symptom improvement in 87% of the treatment group and 10% of the control group (P=0.0007). These early results were supported by several small open label case series published between 2003 and 2009. These studies lacked placebo control but they seemed to confirm the beneficial effects of BoNT across a variety of inclusion criteria and outcome measures including International Prostate Symptom Score (IPSS), QMax, PVR, QoL scores and urodynamic parameters (88-91,94,96,100). The results of these studies are summarized in Table 4.
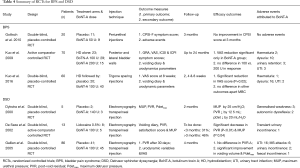
Full table
Based on these results, several large RCTs have been undertaken with conflicting results. Two double-blind, randomized, placebo controlled trials which included approximately 700 patients concluded that there was no significant difference in improvement in IPSS score between placebo and various does of BoNT-A (92,95). A systematic review and meta-analysis which combined all three RCTs (REF) confirmed that the current level 1 evidence does not support any difference in efficacy between BoNT-A injections and placebo (101).
The primary issue from the placebo controlled RCTs is that men with LUTS/BPH appear to have a large placebo response to injection therapy into the prostate. The RCTs have reported a large placebo effect from the sham injection treatment with improvements in IPSS score of up to 25%. This issue is being addressed by the PROTOX study which is a non-inferiority RCT comparing optimal medical treatment with BoNT-A injections. At present, the initial results have been presented in international conferences and are yet to undergo peer review (93) (Table 3).
However, the early result suggests that after 4 months follow-up BoNT-A injections are not inferior to optimised medical treatment based on IPSS score. Moreover, the post hoc analysis of one of the recent RCTs (95) has identified a subgroup of prior α blocker users who did have a significant reduction in IPSS score with BoNT-A 200 U compared to placebo (95). Therefore, BoNT-A prostatic injection may have a role as an alternative to medical treatment or there may be a subset of patients on maximum medical therapy who may benefit from BoNT-A as an adjunct.
Nevertheless, there remains conflicting level 1 evidence and therefore BoNT-A prostatic injection cannot be recommended for routine use in clinical practice until further research is completed. This is supported by a number of international BPH guidelines which have been updated without including BoNT-A injections (102).
AEs
The early studies did not report any significant AEs. The larger high quality randomized control studies reported an AE rate of 30% (92). These were classified as mild or moderate in severity and the most common AEs are haematuria (12.7%) and haematospermia (8.2%). Prostatitis appears to be the most common infection related complication and in one study was higher in the group undergoing BoNT-A injections via the transrectal route (95). Given that there was no significant different in any AE between placebo and treatment arms, it is likely that these are related to the injection procedure rather than BoNT-A treatment.
Future developments
There is ongoing research into alternative mechanisms to deliver BoNT-A in order to improve tolerability of the treatment and reduce AEs. The high molecular weight of BoNT-A (150 kDa) has restricted administration to cystoscopic injection in order to reach the suburothelium, but injections are associated with discomfort, risk of UTI and urinary retention. There have been promising development with liposome enucleated BoNT-A instillations which may provide a mechanism of transporting BoNT-A across the urothelium (103). The early pilot studies in clinical practice have shown encouraging results (104-106). Chuang et al. published on the use of intravesical liposome complex in BPS patients and found a significant decrease in urinary frequency and nocturia compared to baseline (104). Kuo et al. has published a RCT study in OAB patients treated with 80 mg liposomes and 200 U BoNT-A or normal saline, showing similar improvement in frequency and urgency episodes but no significant change in UUI episodes (105). The treatment did not cause any UTIs, raised PVRs or episodes of retention and may be a promising alternative approach to cystoscopic injection.
Conclusions
Over the last decade, BoNT-A has developed into valuable a treatment option for a range of lower urinary tract conditions. Regulatory phase III trials have conclusively demonstrated that for both NDO and OAB, BoNT-A decreases UII, improves urodynamic parameters and increases QoL. There is robust data for long-term efficacy and safety outcomes across multiple treatment cycles.
Other applications remain off-license but there is accumulating level 1 evidence that BoNT-A is beneficial in the treatment of BPS. Its use is likely to grow following recommendation in the AUA guidelines as a fourth line treatment options. In contrast, there is a lack of high quality evidence with DSD and no definite recommendation can be made based on the current evidence. Finally, the results for the treatment of BPH have been variable and recent high quality RCTs have suggested no benefit over placebo so at present it cannot be recommended for routine clinical practice.
Acknowledgements
The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre at Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The authors also acknowledge the support of the MRC Centre for Transplantation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Kreyden OP. Botulinum toxin: from poison to pharmaceutical. In: Kreyden OP, Böni R, Burg G, editors. Hyperhidrosis and Botulinum Toxin in Dermatology. Karger Publishers, 2002:94-100.
- Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980;87:1044-9. [Crossref] [PubMed]
- Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm (Vienna) 2008;115:559-65. [Crossref] [PubMed]
- Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139:919-22. [PubMed]
- Schurch B, Schmid DM, Stöhrer M. Treatment of neurogenic incontinence with botulinum toxin A. N Engl J Med 2000;342:665. [Crossref] [PubMed]
- Radziszewski P, Dobronski P, Borkowski A. Treatment of the non-neurogenic storage and voiding disorders with the chemical denervation caused by botulinum toxin type A: a pilot study. Neurourol Urodyn 2001;20:410-2.
- Zermann DH, Ishigooka M, Schmidt RJ, et al. Trigonum and bladder base injection of botulinum toxin A (BTX) in patients with severe urgency-frequency syndrome refractory to conservative medical treatment and electrical stimulation. Neurourol Urodyn 2001;20:412-3.
- Smith CP, Radziszewski P, Borkowski A, et al. Botulinum toxin a has antinociceptive effects in treating interstitial cystitis. Urology 2004;64:871-5; discussion 875. [Crossref] [PubMed]
- Maria G, Brisinda G, Civello IM, et al. Relief by botulinum toxin of voiding dysfunction due to benign prostatic hyperplasia: results of a randomized, placebo-controlled study. Urology 2003;62:259-64; discussion 264-5. [Crossref] [PubMed]
- Sampaio C, Costa J, Ferreira JJ. Clinical comparability of marketed formulations of botulinum toxin. Mov Disord 2004;19 Suppl 8:S129-36. [Crossref] [PubMed]
- FDA. BOTOX: Approval Label. Supple. 5251. Cited August, 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5232lbl.pdf
- MHRA. BOTOX: Summary of Product Characteristics. Cited August, 2016. Available online: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con208720.pdf
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol 2012;188:2455-63. [Crossref] [PubMed]
- Nambiar A, Lucas M. Chapter 4: Guidelines for the diagnosis and treatment of overactive bladder (OAB) and neurogenic detrusor overactivity (NDO). Neurourol Urodyn 2014;33 Suppl 3:S21-5. [Crossref] [PubMed]
- Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 2006;49:644-50. [Crossref] [PubMed]
- Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther 1980;212:16-21. [PubMed]
- Dong M, Yeh F, Tepp WH, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006;312:592-6. [Crossref] [PubMed]
- Coelho A, Cruz F, Cruz CD, et al. Spread of onabotulinumtoxinA after bladder injection. Experimental study using the distribution of cleaved SNAP-25 as the marker of the toxin action. Eur Urol 2012;61:1178-84. [Crossref] [PubMed]
- Kuo HC. Reduction of urgency severity is associated with long-term therapeutic effect after intravesical onabotulinumtoxin A injection for idiopathic detrusor overactivity. Neurourol Urodyn 2011;30:1497-502. [Crossref] [PubMed]
- Harper M, Popat RB, Dasgupta R, et al. A minimally invasive technique for outpatient local anaesthetic administration of intradetrusor botulinum toxin in intractable detrusor overactivity. BJU Int 2003;92:325-6. [Crossref] [PubMed]
- Leong RK, de Wachter SG, Joore MA, et al. Cost-effectiveness analysis of sacral neuromodulation and botulinum toxin A treatment for patients with idiopathic overactive bladder. BJU Int 2011;108:558-64. [Crossref] [PubMed]
- Popat R, Apostolidis A, Kalsi V, et al. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J Urol 2005;174:984-9. [Crossref] [PubMed]
- Mascarenhas F, Cocuzza M, Gomes CM, et al. Trigonal injection of botulinum toxin-A does not cause vesicoureteral reflux in neurogenic patients. Neurourol Urodyn 2008;27:311-4. [Crossref] [PubMed]
- Karsenty G, Elzayat E, Delapparent T, et al. Botulinum toxin type a injections into the trigone to treat idiopathic overactive bladder do not induce vesicoureteral reflux. J Urol 2007;177:1011-4. [Crossref] [PubMed]
- Pinto R, Lopes T, Frias B, et al. Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur Urol 2010;58:360-5. [Crossref] [PubMed]
- Manecksha RP, Cullen IM, Ahmad S, et al. Prospective randomised controlled trial comparing trigone-sparing versus trigone-including intradetrusor injection of abobotulinumtoxinA for refractory idiopathic detrusor overactivity. Eur Urol 2012;61:928-35. [Crossref] [PubMed]
- Davis NF, Burke JP, Redmond EJ, et al. Trigonal versus extratrigonal botulinum toxin-A: a systematic review and meta-analysis of efficacy and adverse events. Int Urogynecol J 2015;26:313-9. [Crossref] [PubMed]
- Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomized, double-blind, placebo-controlled trial. Eur Urol 2011;60:742-50. [Crossref] [PubMed]
- Ginsberg D, Gousse A, Keppenne V, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol 2012;187:2131-9. [Crossref] [PubMed]
- Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 2010;184:2416-22. [Crossref] [PubMed]
- Rovner E, Kennelly M, Schulte-Baukloh H, et al. Urodynamic results and clinical outcomes with intradetrusor injections of onabotulinumtoxinA in a randomized, placebo-controlled dose-finding study in idiopathic overactive bladder. Neurourol Urodyn 2011;30:556-62. [Crossref] [PubMed]
- Denys P, Le Normand L, Ghout I, et al. Efficacy and safety of low doses of onabotulinumtoxinA for the treatment of refractory idiopathic overactive bladder: a multicentre, double-blind, randomized, placebo-controlled dose-ranging study. Eur Urol 2012;61:520-9. [Crossref] [PubMed]
- Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005;174:196-200. [Crossref] [PubMed]
- Ehren I, Volz D, Farrelly E, et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: a randomized, placebo-controlled, double-blind study. Scand J Urol Nephrol 2007;41:335-40. [Crossref] [PubMed]
- Herschorn S, Gajewski J, Ethans K, et al. Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: a randomized, double-blind trial. J Urol 2011;185:2229-35. [Crossref] [PubMed]
- Ginsberg D, Cruz F, Herschorn S, et al. OnabotulinumtoxinA is effective in patients with urinary incontinence due to neurogenic detrusor overactivity [corrected] regardless of concomitant anticholinergic use or neurologic etiology. Adv Ther 2013;30:819-33. [Crossref] [PubMed]
- Rovner E, Dmochowski R, Chapple C, et al. OnabotulinumtoxinA improves urodynamic outcomes in patients with neurogenic detrusor overactivity. Neurourol Urodyn 2013;32:1109-15. [Crossref] [PubMed]
- Kennelly M, Dmochowski R, Schulte-Baukloh H, et al. Efficacy and safety of onabotulinumtoxinA therapy are sustained over 4 years of treatment in patients with neurogenic detrusor overactivity: Final results of a long-term extension study. Neurourol Urodyn 2017;36:368-75. [Crossref] [PubMed]
- Giannantoni A, Conte A, Proietti S, et al. Botulinum toxin type A in patients with Parkinson's disease and refractory overactive bladder. J Urol 2011;186:960-4. [Crossref] [PubMed]
- Kuo HC. Therapeutic effects of suburothelial injection of botulinum a toxin for neurogenic detrusor overactivity due to chronic cerebrovascular accident and spinal cord lesions. Urology 2006;67:232-6. [Crossref] [PubMed]
- Haylen BT, Freeman RM, Lee J, et al. International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related to native tissue female pelvic floor surgery. Neurourol Urodyn 2012;31:406-14. [Crossref] [PubMed]
- Sahai A, Khan MS, Dasgupta P. Efficacy of Botulinum Toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol 2007;177:2231-6. [PubMed]
- Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol 2008;180:217-22. [Crossref] [PubMed]
- Flynn MK, Amundsen CL, Perevich M, et al. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol 2009;181:2608-15. [Crossref] [PubMed]
- Anger JT, Weinberg A, Suttorp MJ, et al. Outcomes of intravesical botulinum toxin for idiopathic overactive bladder symptoms: a systematic review of the literature. J Urol 2010;183:2258-64. [Crossref] [PubMed]
- Chapple C, Sievert KD, MacDiarmid S, et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomized, double-blind, placebo-controlled trial. Eur Urol 2013;64:249-56. [Crossref] [PubMed]
- Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013;189:2186-93. [Crossref] [PubMed]
- Sievert KD, Chapple C, Herschorn S, et al. OnabotulinumtoxinA 100U provides significant improvements in overactive bladder symptoms in patients with urinary incontinence regardless of the number of anticholinergic therapies used or reason for inadequate management of overactive bladder. Int J Clin Pract 2014;68:1246-56. [Crossref] [PubMed]
- Makovey I, Davis T, Guralnick ML, et al. Botulinum toxin outcomes for idiopathic overactive bladder stratified by indication: lack of anticholinergic efficacy versus intolerability. Neurourol Urodyn 2011;30:1538-40. [Crossref] [PubMed]
- Nitti VW, Ginsberg D, Sievert KD, et al. Durable Efficacy and Safety of Long-Term OnabotulinumtoxinA Treatment in Patients with Overactive Bladder Syndrome: Final Results of a 3.5-Year Study. J Urol 2016;196:791-800. [Crossref] [PubMed]
- Compérat E, Reitz A, Delcourt A, et al. Histologic features in the urinary bladder wall affected from neurogenic overactivity--a comparison of inflammation, oedema and fibrosis with and without injection of botulinum toxin type A. Eur Urol 2006;50:1058-64. [Crossref] [PubMed]
- European Association of Urology. Guidelines on chronic pelvic pain. 2016. Available online: https://uroweb.org/guideline/chronic-pelvic-pain/
- Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015;193:1545-53. [Crossref] [PubMed]
- Mayer R. Interstitial cystitis pathogenesis and treatment. Curr Opin Infect Dis 2007;20:77-82. [Crossref] [PubMed]
- Grover S, Srivastava A, Lee R, et al. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 2011;3:19-33. [Crossref] [PubMed]
- Sant GR, Theoharides TC. The role of the mast cell in interstitial cystitis. Urol Clin North Am 1994;21:41-53. [PubMed]
- Jhang JF, Kuo HC. Botulinum Toxin A and Lower Urinary Tract Dysfunction: Pathophysiology and Mechanisms of Action. Toxins (Basel) 2016;8:120. [Crossref] [PubMed]
- Lucioni A, Bales GT, Lotan TL, et al. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int 2008;101:366-70. [Crossref] [PubMed]
- Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int 2009;104:657-61. [Crossref] [PubMed]
- Mangera A, Andersson KE, Apostolidis A, et al. Contemporary Management of Lower Urinary Tract Disease With Botulinum Toxin A: a systematic review of botox (onabotulinumtoxina) and dysport (abobotulinumtoxinA). Eur Urol 2011;60:784-95. [Crossref] [PubMed]
- Giannantoni A, Costantini E, Di Stasi SM, et al. Botulinum A toxin intravesical injections in the treatment of painful bladder syndrome: a pilot study. Eur Urol 2006;49:704-9. [Crossref] [PubMed]
- Chung SD, Kuo YC, Kuo HC. Intravesical onabotulinumtoxinA injections for refractory painful bladder syndrome. Pain physician 2012;15:197-202. [PubMed]
- Gottsch HP, Miller JL, Yang CC, et al. A pilot study of botulinum toxin for interstitial cystitis/painful bladder syndrome. Neurourol Urodyn 2011;30:93-6. [Crossref] [PubMed]
- Kuo HC, Jiang YH, Tsai YC, et al. Intravesical botulinum toxin-A injections reduce bladder pain of interstitial cystitis/bladder pain syndrome refractory to conventional treatment - A prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial. Neurourol Urodyn 2016;35:609-14. [Crossref] [PubMed]
- Lee CL, Kuo HC. Intravesical botulinum toxin a injections do not benefit patients with ulcer type interstitial cystitis. Pain physician 2013;16:109-16. [PubMed]
- Chancellor MB, Kaplan SA, Blaivas JG. Detrusor-external sphincter dyssynergia. Ciba Found Symp 1990;151:195-206; discussion 207-13. [PubMed]
- Utomo E, Groen J, Blok BF. Surgical management of functional bladder outlet obstruction in adults with neurogenic bladder dysfunction. Cochrane Database Syst Rev 2014.CD004927. [PubMed]
- Burgen AS, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol 1949;109:10-24. [Crossref] [PubMed]
- Apostolidis A, Dasgupta P, Denys P, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol 2009;55:100-19. [Crossref] [PubMed]
- Chen SL, Bih LI, Chen GD, et al. Comparing a transrectal ultrasound-guided with a cystoscopy-guided botulinum toxin a injection in treating detrusor external sphincter dyssynergia in spinal cord injury. Am J Phys Med Rehabil 2011;90:723-30. [Crossref] [PubMed]
- Mahfouz W, Karsenty G, Corcos J. Injection of botulinum toxin type A in the urethral sphincter to treat lower urinary tract dysfunction: review of indications, techniques and results: 2011 update. Can J Urol 2011;18:5787-95. [PubMed]
- Kuo YC, Kuo HC. Botulinum toxin injection for lower urinary tract dysfunction. Int J Urol 2013;20:40-55. [Crossref] [PubMed]
- Wheeler JS Jr, Walter JS, Chintam RS, et al. Botulinum toxin injections for voiding dysfunction following SCI. J Spinal Cord Med 1998;21:227-9. [Crossref] [PubMed]
- Schurch B, Hodler J, Rodic B. Botulinum A toxin as a treatment of detrusor-sphincter dyssynergia in patients with spinal cord injury: MRI controlled transperineal injections. J Neurol Neurosurg Psychiatry 1997;63:474-6. [Crossref] [PubMed]
- Liao YM, Kuo HC. Causes of failed urethral botulinum toxin A treatment for emptying failure. Urology 2007;70:763-6. [Crossref] [PubMed]
- Cui XG, Qu C, Xu DF, et al. Botulinum toxin injection into urethral external sphincter combined with oral baclofen in treatment of patients with detrusor-external sphincter dyssynergia after spinal cord injury. Academic Journal of Second Military Medical University 2007;28:875-7.
- Petit H, Wiart L, Gaujard E, et al. Botulinum A toxin treatment for detrusor-sphincter dyssynergia in spinal cord disease. Spinal cord 1998;36:91-4. [Crossref] [PubMed]
- Dykstra DD, Sidi AA. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: a double-blind study. Arch Phys Med Rehabil 1990;71:24-6. [PubMed]
- de Sèze M, Petit H, Gallien P, et al. Botulinum a toxin and detrusor sphincter dyssynergia: a double-blind lidocaine-controlled study in 13 patients with spinal cord disease. Eur Urol 2002;42:56-62. [Crossref] [PubMed]
- Gallien P, Reymann JM, Amarenco G, et al. Placebo controlled, randomized, double blind study of the effects of botulinum A toxin on detrusor sphincter dyssynergia in multiple sclerosis patients. J Neurol Neurosurg Psychiatry 2005;76:1670-6. [Crossref] [PubMed]
- Mehta S, Hill D, Foley N, et al. A meta-analysis of botulinum toxin sphincteric injections in the treatment of incomplete voiding after spinal cord injury. Arch Phys Med Rehabil 2012;93:597-603. [Crossref] [PubMed]
- Kuo HC. Satisfaction with urethral injection of botulinum toxin A for detrusor sphincter dyssynergia in patients with spinal cord lesion. Neurourol Urodyn 2008;27:793-6. [Crossref] [PubMed]
- Chuang YC, Huang CC, Kang HY, et al. Novel action of botulinum toxin on the stromal and epithelial components of prostate gland. J Urol 2006;175:1158-63. [Crossref] [PubMed]
- Pennefather JN, Lau WA, Mitchelson F, et al. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol 2000;20:193-206. [Crossref] [PubMed]
- Ruggieri MR, Colton MD, Wang P, et al. Human prostate muscarinic receptor subtypes. J Pharmacol Exp Ther 1995;274:976-82. [PubMed]
- Luthin GR, Wang P, Zhou H, et al. Role of m1 receptor-G protein coupling in cell proliferation in the prostate. Life Sci 1997;60:963-8. [Crossref] [PubMed]
- Witte LP, Chapple CR, de la Rosette JJ, et al. Cholinergic innervation and muscarinic receptors in the human prostate. Eur Urol 2008;54:326-34. [Crossref] [PubMed]
- Chuang YC, Chiang PH, Huang CC, et al. Botulinum toxin type A improves benign prostatic hyperplasia symptoms in patients with small prostates. Urology 2005;66:775-9. [Crossref] [PubMed]
- Chuang YC, Tu CH, Huang CC, et al. Intraprostatic injection of botulinum toxin type-A relieves bladder outlet obstruction in human and induces prostate apoptosis in dogs. BMC Urol 2006;6:12. [Crossref] [PubMed]
- Brisinda G, Cadeddu F, Vanella S, et al. Relief by botulinum toxin of lower urinary tract symptoms owing to benign prostatic hyperplasia: early and long-term results. Urology 2009;73:90-4. [Crossref] [PubMed]
- Silva J, Pinto R, Carvalho T, et al. Intraprostatic Botulinum Toxin Type A injection in patients with benign prostatic enlargement: duration of the effect of a single treatment. BMC Urol 2009;9:9. [Crossref] [PubMed]
- McVary KT, Roehrborn CG, Chartier-Kastler E, et al. A multicenter, randomized, double-blind, placebo controlled study of onabotulinumtoxinA 200 U to treat lower urinary tract symptoms in men with benign prostatic hyperplasia. J Urol 2014;192:150-6. [Crossref] [PubMed]
- Delongchamps NB, Descazeaud A, Benard A, et al. A randomized clinical trial comparing prostatic injection of botulinum neurotoxin type A (Botox®) to optimized medical therapy in patients with BPH-related LUTS: End-of-study results of the PROTOX trial. European Urology Supplements 2016;15:e1079a. [Crossref]
- Kuo HC. Prostate botulinum A toxin injection--an alternative treatment for benign prostatic obstruction in poor surgical candidates. Urology 2005;65:670-4. [Crossref] [PubMed]
- Marberger M, Chartier-Kastler E, Egerdie B, et al. A Randomized Double-blind Placebo-controlled Phase 2 Dose-ranging Study of OnabotulinumtoxinA in men with benign prostatic hyperplasia. Eur Urol 2013;63:496-503. [Crossref] [PubMed]
- Chuang YC, Chiang PH, Yoshimura N, et al. Sustained beneficial effects of intraprostatic botulinum toxin type A on lower urinary tract symptoms and quality of life in men with benign prostatic hyperplasia. BJU Int 2006;98:1033-7; discussion 1337. [Crossref] [PubMed]
- Grummet JP, Weerakoon M, Huang S, et al. Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int 2014;114:384-8. [PubMed]
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol 2013;64:876-92. [Crossref] [PubMed]
- Crawford ED, Hirst K, Kusek JW, et al. Effects of 100 and 300 units of onabotulinum toxin A on lower urinary tract symptoms of benign prostatic hyperplasia: a phase II randomized clinical trial. J Urol 2011;186:965-70. [Crossref] [PubMed]
- Reddy S, Chawla A, Thomas J. Botulinum toxin in high-risk BPH patients in retention. Indian J Urol 2008;24:276. [PubMed]
- Shim SR, Cho YJ, Shin IS, et al. Efficacy and safety of botulinum toxin injection for benign prostatic hyperplasia: a systematic review and meta-analysis. Int Urol Nephrol 2016;48:19-30. [Crossref] [PubMed]
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU Guidelines on the Assessment of Non-neurogenic Male Lower Urinary Tract Symptoms including Benign Prostatic Obstruction. Eur Urol 2015;67:1099-109. [Crossref] [PubMed]
- Chuang YC, Tyagi P, Huang CC, et al. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J Urol 2009;182:786-92. [Crossref] [PubMed]
- Chuang YC, Lee WC, Lee WC, et al. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol 2009;182:1393-400. [Crossref] [PubMed]
- Kuo HC, Liu HT, Chuang YC, et al. Pilot study of liposome-encapsulated onabotulinumtoxina for patients with overactive bladder: a single-center study. Eur Urol 2014;65:1117-24. [Crossref] [PubMed]
- Chuang YC, Kaufmann JH, Chancellor DD, et al. Bladder Instillation of liposome encapsulated onabotulinumtoxina improves overactive bladder symptoms: a prospective, multicenter, double-blind, randomized trial. J Urol 2014;192:1743-9. [Crossref] [PubMed]


