Improvement of motility after culture of testicular spermatozoa: the effects of incubation timing and temperature
Introduction
Almost 10% to 20% of all male infertile cases are suffering with azoospermia (1,2). Testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI) are performed for treatment of men diagnosed with obstructive or non-obstructive azoospermia (OA) (3). Testicular spermatozoa are, however, immature physiologically and often are immotile or with finely twitching movement instantly after biopsy (4). Improvement of testicular sperm motility after in-vitro culture is the good strategy in azoospermic cases (5). Some procedures for in vitro maturation of human testicular spermatozoa have been proposed by researchers (6).
In vivo sperm maturation is the process where spermatozoa access to the potential for progressive motility and fertilization. It is the next phase of spermiation stage that spermatozoa are released from the seminiferous tubules and reach the epididymal site (7). Passage of human spermatozoa through epididymis lasts about 10 days which coincides with structural modifications and changes in the lipid and protein composition of the sperm membrane (8,9). It finally leads to their achievement of progressive motility (10,11).
Immediately after testicular biopsy, finding of sufficient numbers of motile sperm cells in azoospermia patients is very difficult. There are some chemical products that are stimulator for in-vitro improvements of the testicular sperm motility (12). These are pentoxifylline (13) 2-deoxyadenosine (12) and caffeine (14) that induce variations in sperm cyclic AMP contents. However, these drugs may be toxic to the spermatozoa and the resulting embryos in assisted reproduction strategy (15). Also, when spermatozoa are exposed to these products, motility exhibits only for short time, and then become immotile permanently, because depletion of sperm’s metabolic resources occurs rapidly (12). Although, some studies reported that in-vitro culturing of testicular samples can increase the number of motile spermatozoa, but there are no guidelines to suggest the suitable incubation time or temperature used to improve sperm motility in TESE situation. Therefore, determination of appropriate incubation time and temperature for the testicular sperms before ICSI or cryopreservation procedure to yield a proper extracellular environment is critical (16). Therefore, this study was designed to assess the role of different incubation time intervals and temperature on motility feature of spermatozoa extracted from testes biopsies in azoospermia cases.
Methods
Patients
In this prospective study, the subjects were selected between September 2015 and October 2016. Testicular biopsies were obtained from 27 men with OA referred to our infertility center for diagnostic purpose. We collected the specimens from discarded TESE samples for the study. The mean age of the men was 39 years (range, 29–49 years). Informed consent was obtained from each patient and the project was approved by Ethics committee of authors’ institution (IR.SSU.RSI.REC.1394.30).
Testicular biopsy preparation
Briefly, human testicular tissue was obtained surgically (TESE) from patients with OA. The samples were minced mechanically and dissected into small pieces using needle in a sterile Petri dish. The tissue samples were kept moist with Hams F10 medium (Sigma Chemical Co., St Louis, MO, USA) during dissection. Initial examination for the presence of spermatozoa was performed under an inverted phase-contrast microscope (Olympus, Tokyo, Japan). The suspension was then transferred to a test tube, if spermatozoa were noticed in Petri dish. TESE spermatozoa were often accompanied with red blood cells, debris, testicular cells and a large number of dead or immotile spermatozoa that have detrimental effects on existing motile spermatozoa. The samples were centrifuged with RBC lysis buffer for 5 min at 300 g to eliminate these cells. The pellets were then re-suspend in 3 mL of Hams F10 and centrifuged at 500 g for 10 min to wash off the RBC lysis buffer, and to preserve the integrity of the other testicular cells. Finally, the pellet was resuspended in 0.5 mL of Hams F10+20% HSA medium (In Vitro Care Inc., San Diego, CA, USA) (17).
In vitro incubation of testicular tissue
Ham’s F10+20% HSA media is considered as an appropriate medium for sperm culture (18). After preparation, drops of 10 µL were placed in a Petri dish and covered with mineral oil (Sage Biopharma, Bedminster, NJ, USA). Individual spermatozoon was observed carefully with a high magnification under the microscope to evaluate any sign of sperm motility, such as twitching of the head or tail. A total of 100 spermatozoa were observed in all microdrops, and the motility was assessed at day 0 (D0) under an inverted microscope. Then, the suspension was divided into two fractions. The first part consisting of sample that was cultured for 3 days at 37 °C in 6% CO2, 5% O2 balance N2. Because, 37 °C is high temperature compared with the physiologic temperature for testes tissue, we cultured the second part for 3 days at room temperature (RT) of 25 °C.
About 0.5 mL of the upper layer of Hams F10+20% HSA was changed with the same volume of the fresh medium daily. The culturing samples were assessed daily for percentage of motile spermatozoa using the same method as the initial collection by an inverted phase-contrast microscope (Nikon Diaphot 300; UK Ltd., Kingston). The PH and temperature of the culture medium and incubator were controlled daily and maintained throughout the study.
Statistical analysis
SPSS software package was used for data analysis. The Paired Student’s t-test was performed to compare the differences in the parametric, and Mann Whitney U test was used in non-parametric groups. Statistical significance was set at P≤0.05. The data showed as mean ± SD.
Results
The findings indicated that very few spermatozoa demonstrated twitching tail in samples from collecting biopsy tissue on D0. After culture to D1, a maximum range of sperm cells began demonstration of twitching or wavy flagellar movement in both groups. Even, spermatozoa in some of the cases were seen to be swimming at the periphery of the culture droplets. However, the numbers of motile spermatozoa in incubator culturing condition at D1 were significantly lower than RT condition (67.85±13.2 vs. 76.3±10.5; P≤0.05). With further culturing, the spermatozoa lost their progressive motility, but maintained the twitching motility after 48 h of culturing (D2). However, when in-vitro culturing was continued for three days (D3), the sperm motility rate was decreased significantly in both groups (Table 1).
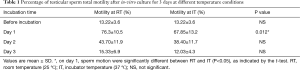
Full table
Discussion
Successful pregnancies and healthy babies were achieved after TESE followed by ICSI (19,20). In some countries, such as the United Kingdom, practice guidelines stipulate that only documented motile sperms can be injected into oocytes. According to these guidelines, only motile sperms have to be injected into MII oocytes in IVF facilities. Other reports declared that the injection of immotile sperm to the oocyte may lead to the failure of the fertilization (21,22). Motility is a safe, practical and necessary method to estimate the sperm viability in ICSI program (4). However, it was reported that only >3% of the testicular spermatozoa are motile after a biopsy (15). Some studies suggested the testicular sperm culturing technique to stimulate the sperm motility (23-25). The exact mechanism of improving the motion of testicular sperms after in vitro culture is still not clear. In the current study, our samples were prepared with simple wash method before culturing and all of the testicular cells, such as sertoli, leydig and germ cells were present during the culturing along with the sperm cells. The interaction between testicular somatic and germ cells and even the interaction among germ cells may enhance the sperm maturation process, presumably by providing a co-culturing system (12). It was suggested that having access to a progressive motility is the sign of sperm maturation (5,26). Another factor in the beginning of sperm motility may be due to the elimination of inhibitory factors with simple wash before culturing. The activation or synthesis of essential factors assists in motility improvement (12). Gradual degeneration of the testicular somatic cells after a long-term culturing may decrease the sperm motility.
Adding chemical components, including 2-deoxyadenosine and pentoxifylline (27) or hypo-osmotic swelling test (HOST) (28,29) can stimulate the motility or viability of the testicular sperms in vitro condition. In conditions that only twitching spermatozoa are available, in vitro culturing is more effective. Using motile spermatozoa after this method can be a preferable optimal alternative, rather than chemical components and HOST. We found that a small number of sperms has a typical motile immediately after testicular biopsy analysis, but after culturing them in RT condition, sperm motility was significantly increased in comparison with culturing at 37 °C after 24 h. It should be noted that motile spermatozoa were still present in both conditions at D2 of culture, but their motility begins to decline significantly.
Although, Wood et al. proved that there is not a difference in motility between immediate and cultured testicular sperms (30), some studies have confirmed our results (24,31-33). Since, after 24–48 h of culturing, adequate motile spermatozoa may exist in the culture media, a period of 24–48 h as an optimal time for the development of motility is suggested (4,24). They suggested that the sperm testicular biopsy is achieved 1 or 2 days before the oocyte retrieval. Also, Bin et al. showed that this finding was eligible in the frozen-thawed TESE specimens. They reported that culturing frozen-thawed TESE samples for 24 h, presented a similar motility with fresh TESE samples (4). The sperm in vitro culturing approach is associated with aging in long-term culturing. Due to the presence of high unsaturated fatty acid in its membrane and deficiency of the antioxidant in its cytoplasm (34), sperms may be vulnerable to harmful effects of reactive oxygen species (ROS) which may lead to sperm DNA damages (12). Recently, Schiewe concluded that the elevated ROS and DNA fragmentation index (DFI) after in vitro culturing are not harmful, and they may be due to the presence of dead sperms in solution and using them does not have any impaired clinical outcomes (32). Indeed, ROS production in small amounts, the by-product of electron transfer chain in sperm cells, is necessary for sperm vital activities (35). However, if these products aggregate during the culturing, they will gradually become toxic to the spermatozoa (36).
The applied media and the culturing duration are two main factors in sperm preparation prior to ART techniques. Hams F10 is a common media in ART laboratories that was used for sperm culturing. Also, adding serum supplementation may help enrich the culturing media. If sperm culturing is performed in a simple and unsupplemented media (23,24,37) or the media is not changed or gassed with CO2 (31), it may result in the deficiency of energy and nutritious substrates. Using suitable and changeable mediums can be supportive for sperm maturation. In this study, we cultured the samples in Hams F10+HSA that are an isotonic medium to ensure sperm survival by avoiding osmotic shock and pH alterations, and it provides the optimal buffering capacity. Adding serum to the culture media acts as a powerful antioxidant with an important role in preserving sperms from oxidative stress-induced damages, helping its motility and preventing lipid peroxidation (38). Also, proteins of HSA are a nutrient in the culture media; it seems that their existence has some effects on the sperm motility (39).
In the current study, we changed the culturing medium daily, which caused the preservation of sperm motility. Our data showed that the majority of testicular sperms acquired motility and became mature after culturing, if they were normal. A decline in motion after prolong culturing may be due to the sperm cells death that may be induced by necrosis and apoptosis. Also, the evaluation of testicular sperm with electron microscope reported a structural anomaly in midpiece, which disturbs the motility, even after in vitro culture (12).
There is a main concern that incubator temperature is beyond the physiologic temperature of testis function and sperm culturing at low temperature may offer a good result in ART programs. Although the spermatozoa culturing at 37 °C is a routine approach in ART laboratories, we found that testicular sperms enhanced their motility better at RT in comparison with culturing at 37 °C. Some sperm functions, like capacitation are temperature dependent processes and the cellular mechanisms that involved in these functions, can be controlled by culturing temperature. Some studies demonstrated that sperm culturing at RT caused a temporary “quiescent” state in the capacitation process (40). Regarding to this feature, sperm culturing at RT may be beneficial for delayed treatment of infertility, especially in cases with azoospermia. It is concluded that testicular sperm culturing at RT for 24 h was appropriate to improve sperm motion in azoospermic patients. Long-term incubation or suboptimal conditions may damage the sperm motion and increase the structural/chromosomal abnormalities (41). Further studies are needed to evaluate the sperm DNA integrity after in-vitro culture of TESE cases.
Acknowledgements
The authors would like to thank the technicians of the Andrology laboratory for their assistance.
Funding: The funding of this research was given by the institute “Research and Clinical Center for Infertility, Yazd, Iran”.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consent was obtained from each patient and the project was approved by Ethics committee of authors’ institution (IR.SSU.RSI.REC.1394.30) and written informed consent was obtained from all patients.
References
- Coetzee K, Ozgur K, Berkkanoglu M, et al. Reliable single sperm cryopreservation in Cell Sleepers for azoospermia management. Andrologia 2016;48:203-10. [Crossref] [PubMed]
- Bocca S, Moussavi V, Brugh V, et al. ICSI outcomes in men undergoing TESE for azoospermia and impact of maternal age. Andrologia 2017;49. [Crossref] [PubMed]
- Desch L, Bruno C, Herbemont C, et al. Impact on ICSI outcomes of adding 24 h of in vitro culture before testicular sperm freezing: a retrospective study. Basic Clin Androl 2015;25:1. [Crossref] [PubMed]
- Wu B, Wong D, Lu S, et al. Optimal use of fresh and frozen-thawed testicular sperm for intracytoplasmic sperm injection in azoospermic patients. J Assist Reprod Genet 2005;22:389-94. [Crossref] [PubMed]
- Zhu J, Tsirigotis M, Pelekanos M, et al. In-vitro maturation of human testicular spermatozoa. Hum Reprod 1996;11:231-2. [Crossref] [PubMed]
- Zhu J, Meniru GI, Craft I. In vitro maturation of human testicular sperm in patients with azoospermia. J Assist Reprod Genet 1997;14:361-3. [Crossref] [PubMed]
- Moore HD, Akhondi MA. In vitro maturation of mammalian spermatozoa. Rev Reprod 1996;1:54-60. [Crossref] [PubMed]
- Sullivan R, Mieusset R. The human epididymis: its function in sperm maturation. Hum Reprod Update 2016;22:574-87. [Crossref] [PubMed]
- Dacheux JL, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction 2013;147:R27-R42. [Crossref] [PubMed]
- Jones RC, Murdoch RN. Regulation of the motility and metabolism of spermatozoa for storage in the epididymis of eutherian and marsupial mammals. Reprod Fertil Dev 1996;8:553-68. [Crossref] [PubMed]
- Morton BE, Sagadraca R, Fraser C. Sperm motility within the mammalian epididymis: species variation and correlation with free calcium levels in epididymal plasma. Fertil Steril 1978;29:695-8. [Crossref] [PubMed]
- Angelopoulos T, Adler A, Krey L, et al. Enhancement or initiation of testicular sperm motility by in vitro culture of testicular tissue. Fertil Steril 1999;71:240-3. [Crossref] [PubMed]
- Archer SL, Roudebush WE. Enhancement of sperm motility using pentoxifylline and platelet-activating factor. Methods Mol Biol 2013.241-5. [Crossref] [PubMed]
- Nabavi N, Todehdehghan F, Shiravi A. Effect of caffeine on motility and vitality of sperm and in vitro fertilization of outbreed mouse in T6 and M16 media. Iran J Reprod Med 2013;11:741. [PubMed]
- Lacham-Kaplan O, Trounson A. The effects of the sperm motility activators 2-deoxyadenosine and pentoxifylline used for sperm micro-injection on mouse and human embryo development. Hum Reprod 1993;8:945-52. [Crossref] [PubMed]
- Morris DS, Davis DE, Dunn RL, et al. Ideal culture time for improvement in sperm motility from testicular aspirates of azoospermic men. Fertil Steril 2006;86:S105. [Crossref]
- Janosek-Albright KJ, Schlegel PN, Dabaja AA. Testis sperm extraction. Asian J Urol 2015;2:79-84. [Crossref]
- Morris DS, Dunn RL, Schuster TG, et al. Ideal culture time for improvement in sperm motility from testicular sperm aspirates of men with azoospermia. J Urol 2007;178:2087-91. [Crossref] [PubMed]
- Thornhill J, Fanning D, Davis NF, et al. Testicular sperm extraction and intracytoplasmic sperm injection: outcomes in a specialist fertility centre. Ir Med J 2015;108:263-5. [PubMed]
- Hessel M, Robben J, D'Hauwers KW, et al. The influence of sperm motility and cryopreservation on the treatment outcome after intracytoplasmic sperm injection following testicular sperm extraction. Acta Obstet Gynecol Scand 2015;94:1313-21. [Crossref] [PubMed]
- Nijs M, Vanderzwalmen P, Vandamme B, et al. Andrology: Fertilizing ability of immotile spermatozoa after intracytoplasmic sperm injection. Hum Reprod 1996;11:2180-5. [Crossref] [PubMed]
- Liu J, Nagy Z, Joris H, et al. Analysis of 76 total fertilization failure cycles out of 2732 intracytoplasmic sperm injection cycles. Hum Reprod 1995;10:2630-6. [Crossref] [PubMed]
- Balaban B, Urman B, Sertac A, et al. In-vitro culture of spermatozoa induces motility and increases implantation and pregnancy rates after testicular sperm extraction and intracytoplasmic sperm injection. Hum Reprod 1999;14:2808-11. [Crossref] [PubMed]
- Hu Y, Maxson WS, Hoffman DI, et al. Clinical application of intracytoplasmic sperm injection using in vitro cultured testicular spermatozoa obtained the day before egg retrieval. Fertil Steril 1999;72:666-9. [Crossref] [PubMed]
- Lawrence C, Byun J, Chow V, et al. Improvement of sperm motility in surgically retrieved testicular sperm (TESE) samples with in vitro culture. Fertil Steril 2014;102:e98. [Crossref]
- Moore HD, Curry MR, Penfold LM, et al. The culture of human epididymal epithelium and in vitro maturation of epididymal spermatozoa. Fertil Steril 1992;58:776-83. [Crossref] [PubMed]
- Taşdemir I, Taşdemir M, Tavukçuoǧlu Ş. Effect of pentoxifylline on immotile testicular spermatozoa. J Assist Reprod Genet 1998;15:90-2. [Crossref] [PubMed]
- Verheyen G, Joris H, Crits K, et al. Comparison of different hypo-osmotic swelling solutions to select viable immotile spermatozoa for potential use in intracytoplasmic sperm injection. Hum Reprod Update 1997;3:195-203. [Crossref] [PubMed]
- Sallam H, Farrag A, Agameya A, et al. The use of a modified hypo-osmotic swelling test for the selection of viable ejaculated and testicular immotile spermatozoa in ICSI. Hum Reprod 2001;16:272-6. [Crossref] [PubMed]
- Wood S, Sephton V, Searle T, et al. Effect on clinical outcome of the interval between collection of epididymal and testicular spermatozoa and intracytoplasmic sperm injection in obstructive azoospermia. J Androl 2003;24:67-72. [PubMed]
- Edirisinghe WR, Junk SM, Matson PL, et al. Case report: Changes in motility patterns during in-vitro culture of fresh and frozen/thawed testicular and epididymal spermatozoa: implications for planning treatment by intracytoplasmic sperm injection. Hum Reprod 1996;11:2474-6. [Crossref] [PubMed]
- Schiewe MC, Rothman C, Spitz A, et al. Validation-verification of a highly effective, practical human testicular tissue in vitro culture-cryopreservation procedure aimed to optimize pre-freeze and post-thaw motility. J Assist Reprod Genet 2016;33:519-28. [Crossref] [PubMed]
- Lidor C, McDonald JW, Roggli VL, et al. Wear particles in bilateral internal iliac lymph nodes after loosening of a painless unilateral cemented total hip arthroplasty. J Urol 1996;156:1775-6. [Crossref] [PubMed]
- Nabi A, Khalili M, Halvaei I, et al. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia 2014;46:374-9. [Crossref] [PubMed]
- Hamada A, Esteves SC, Agarwal A. editors. Origin and Pathophysiology. Varicocele and Male Infertility. Springer International Publishing, 2016:5-17.
- Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev 1995;7:659-68. [Crossref] [PubMed]
- Liu J, Tsai YL, Katz E, et al. Outcome of in-vitro culture of fresh and frozen-thawed human testicular spermatozoa. Hum Reprod 1997;12:1667-72. [Crossref] [PubMed]
- Sikka SC. Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J Androl 2004;25:5-18. [Crossref] [PubMed]
- Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl 2005;26:459-69. [Crossref] [PubMed]
- Marín-Briggiler CI, Tezón JG, Miranda PV, et al. Effect of incubating human sperm at room temperature on capacitation-related events. Fertil Steril 2002;77:252-9. [Crossref] [PubMed]
- Munné S, Estop AM. Chromosome analysis of human spermatozoa stored in vitro. Hum Reprod 1993;8:581-6. [Crossref] [PubMed]


