Utility of testing sperm DNA fragmentation: an all-in-one diagnostic tool to address a multi-pronged clinical problem
In a comprehensive clinical overview, Agarwal et al. (1) nicely put in perspective (I) various laboratory techniques used for testing sperm DNA fragmentation (SDF), a commonly encountered aberration in the sub-fertile/infertile male, (II) underscore the importance of careful assessment of this parameter in the context of several clinical scenarios of reproductive failure, and (III) recommend the utility of these techniques in identifying the underlying cause with emphasis on its particular relevance to clinical varicoceles in men.
Caution should be exercised to rule out the possibility of interactive effects of multiple factors contributing to SDF before implicating a sole causality since a variety of etiological factors have been noted in animal studies associating them with seminiferous epithelial degeneration ultimately inducing SDF. For example, it is not uncommon to find SDF in equine seminal ejaculates containing bacteria (indicating ongoing subclinical infections of reproductive tract). Typically, sperm in these samples manifest dysplastic acrosomes and premature acrosomal exocytosis possibly resulting from altered milieu of seminal plasma which might contribute to chromatin decondensation and breakage (2,3).
While controlled studies exposing laboratory animals to known reproductive toxicants have been shown to disrupt spermiogenesis [(4); see primary references therein], routine veterinary andrology evaluations of domestic animals also reveal similar lesions in sperm nuclear integrity and morphology (2,3) suggesting the possibility that these animals had been subjected to unintended exposures to comparable environmental toxicants or to therapeutic interventions such as administration of steroids. Salient features of these ultrastructural lesions include interruption of nuclear condensation (e.g., abnormal proliferation of microtubules invading spermatid nucleus during spermiogenesis) and chromatin breakdown (e.g., a variety of nuclear inclusions containing membranous vesicles possibly of glycosylation end products leading to oxidative stress). These subtle lesions are described and presented in Figure 1.
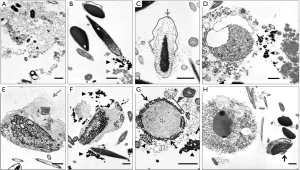
Using a single cytological technique, it is possible to characterize the pattern of SDF, i.e., whether SDF results from impaired chromatin condensation or premature chromatin decondensation and/or breakdown. That dispersed broken down chromatin observed in semen smears at light microscopic level could result from either of the aforementioned situations is illustrated in Figure 2. This diagnostic tool, unlike the SDF testing methods described in this review, not only facilitates visualization of the pattern of SDF at an ultrastructural level unambiguously but also helps in critical evaluation of pivotal organelles of sperm involved in accomplishing fertilization. In addition, it facilitates exploration of any evidence of possible etiologies, e.g., subclinical infections, exposures to pharmaceuticals and environmental toxicants that cause unique lesions.
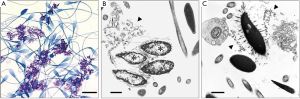
This technique utilizes a sample of seminal ejaculate as biopsy material. Briefly, a semen smear from a freshly collected ejaculate is examined using phase-contrast or differential interference contrast microscopy and then an aliquot of the sample is fixed, centrifuged, and the cell pellet is processed for transmission electron microscopy for critical evaluation of sperm organelles as well as other constituents in the ejaculate such as cells and cellular debris denuded from reproductive tract along with any infectious agents (5,6). Thus this diagnostic technique takes an all-in-one approach to assimilate information otherwise possible only by a battery of assays. Although this procedure at its face-value appears expensive as it requires an electron microscopy facility, it is cost-effective for a dedicated andrology laboratory in providing a comprehensive, critical evaluation of a semen sample especially in clinical situations such as those discussed in this well-written review.
Acknowledgements
Technical assistance of Carol Moeller and Jennifer Palmer is gratefully acknowledged.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Veeramachaneni DN, Sawyer HR. Use of semen as biopsy material for assessment of health status of the stallion reproductive tract. Vet Clin North Am Equine Pract 1996;12:101-10. [Crossref] [PubMed]
- Veeramachaneni DN, Moeller CL, Sawyer HR. Sperm morphology in stallions: ultrastructure as a functional and diagnostic tool. Vet Clin North Am Equine Pract 2006;22:683-92. [Crossref] [PubMed]
- Veeramachaneni DN. Ultrastructural evaluation of semen to assess effects of exposure to toxicants. Toxicol Pathol 2012;40:382-90. [Crossref] [PubMed]
- Veeramachaneni DN, Moeller CL, Pickett BW, et al. On processing and evaluation of equine seminal samples for cytopathology and fertility assessment: the utility of electron microscopy. J Equine Vet Sci 1993;13:207-15. [Crossref]
- Veeramachaneni DN. Electron Microscopy of Semen. In: Dascanio JJ, McCue PM. editors. Equine Reproductive Procedures. Hoboken, NJ: John Wiley & Sons, Inc., 2014;Chapter 120:394-5.


