Guidelines of guidelines: a review of urethral stricture evaluation, management, and follow-up
Introduction
An urethral stricture is an abnormal narrowing of the urethra resulting from fibrosis in the surrounding corpus spongiosum (1). The most common etiology of urethral strictures is idiopathic, followed by iatrogenic causes, including transurethral resection, urethral catheterization, prostate cancer treatments, and previous hypospadias surgery (2). Urethral strictures are known sequela of pelvic and perineal trauma (1,2). The annual costs due to urethral strictures in the United States are estimated to be about $200 million dollars with approximately 5,000 new inpatient visits yearly (3). In the United Kingdom, it is estimated that 700 urethroplasties and 16,000 urethrotomy and dilation procedures are performed annually (4). The costs associated with urethral strictures are compounded by patient morbidity, including urinary tract infections (UTIs), incontinence, and stricture recurrence (2). Recently, the American Urological Association (AUA) released guidelines on the evaluation, management, and follow-up of urethral strictures (5). Prior to this, the only other professional society to put forth guidelines on urethral strictures was Société Internationale d’Urologie (SIU) in 2010 (6). Our objective is to report a side-by-side comparative review summarizing the AUA and SIU guidelines for the evaluation, management, and follow-up of urethral stricture disease.
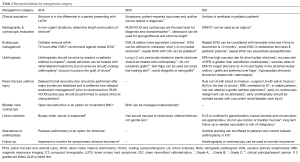
Strength of recommendations is stratified according to letter grade that corresponds to the level of evidence provided by the literature (Table 1). Where available, the grade of recommendation is given. Both AUA and SIU use this scale (7) (Table 2). Grade A recommendations are derived from level 1 evidence or a large body of level two evidence. Level 1 evidence corresponds to meta-analysis of randomized control trials (RTCs), a single good-quality RTC, or very strong studies involving a single treatment option. Level 2 evidence is based on low-quality RCTs (less than 80% follow up) or meta-analysis of prospective cohort studies. Grade B recommendations are drawn from level 2 or 3 evidence or majority evidence from RTCs. Level 3 evidence is based on appropriately matched retrospective case-control or case series studies. Grade C recommendations are drawn from level 4 evidence. Level 4 evidence is expert opinion corresponding to basic physiological or anatomical principals, or bench research (6). Clinical principal is based on widely agreed opinion among urologists that may or may not be evidence based. Expert opinion is a derived from consensus agreement among experts based on clinical training, experience, or knowledge (6,7).
Full table
Full table
Evaluation of urethral strictures
Clinical evaluation
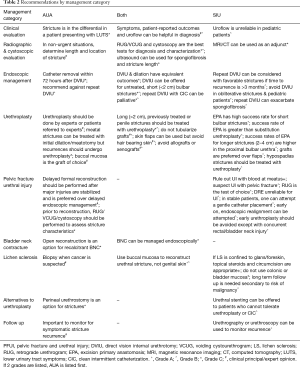
Presenting symptoms of urethral strictures often include decreased urinary stream, incomplete emptying, dysuria, UTI, and/or a rising post void residual (PVR) (5). Other urological conditions, including bladder outlet obstruction from prostatic enlargement, may mimic symptoms of urethral strictures. Common risk factors for developing an urethral stricture include any transurethral surgery, pelvic and perineal trauma, urethral catheterization, prostate cancer treatment, a history of hypospadias repair, and/or lichen sclerosus (LS) (2,8). The AUA guidelines recommend considering an urethral stricture in the differential diagnosis when any male patient presents with the symptoms listed above (AUA; Grade C). The SIU guidelines do not directly comment on the differential diagnosis of urethral stricture disease. Both guidelines recommend the use of patient reported measures, such as the American Urological Association Symptom Index (AUA-SI) and uroflowmetry to evaluate a suspected urethral stricture (AUA; clinical principle) (SIU; Grade B). In particular, the presence of a reduced peak flow (9) places a patient at higher risk for an urethral stricture and further imaging is then indicated (AUA; clinical principle). AUA-SI should be used as an adjunct measure in the initial diagnosis and should not be used alone to diagnose or exclude a stricture (SIU; Grade C).
Radiographic and endoscopic evaluation
Typical options for evaluation of urethral stricture include urethroscopy, retrograde urethrography (RUG), voiding cystourethrography (VCUG), and ultrasound. The AUA guidelines state that radiographic or endoscopic evidence is necessary to make the diagnosis of an urethral stricture, however they do not imply superiority of one individual test to make a definitive diagnosis (AUA; Grade C). The SIU guidelines advocate for a RUG (SIU; Grade A), VCUG (SIU; Grade B), cystoscopy (SIU; Grade A) and/or ultrasound (SIU; Grade C). A RUG generally allows the physician to identify the location and length of the stricture and shows the presence of other urethral pathology such as fistulas or calculi (10). While the AUA reports that RUG is the study of choice for urethral stricture diagnosis, it is highly operator-dependent (11). Cystoscopy is the most specific procedure for the diagnosis of an urethral stricture (12) (SIU; Grade A); however, it is limited as stricture length cannot be determined.
Ultrasound may be used to determine the extent of spongiofibrosis and absolute stricture length (13) (AUA; Grade C), but its use is recommended in conjunction with RUG for preoperative staging and length assessment of anterior urethral strictures (SIU; Grade C). SIU guidelines also mention the use of magnetic resonance imaging (MRI) and computed tomography (CT) as useful adjuncts for the evaluation of urethral stricture, particularly in the setting of pelvic fracture (SIU; Grade C). The AUA guidelines add that in a setting where non-urgent intervention is appropriate, determining length and location of the urethral stricture is recommended (AUA; expert opinion).
Surgical management of urethral stricture
Endoscopic management
Both the AUA (AUA; Grade C) and the SIU (SIU; Grade C) state that both dilation and direct visual internal urethrotomy (DVIU) can be offered in the management of urethral strictures, and the SIU states that both urethral dilation and DVIU have equivalent clinical efficacy (14) (SIU; Grade B). Laser urethrotomy provides no advantage over cold knife procedures and is more costly, therefore laser procedures are not recommended (SIU; Grade A).
DVIU or dilation has a success rate of 50–60% when used as an initial procedure to treat urethral strictures of less than or equal to 2 cm (14,15). Both AUA (AUA; Grade C) and SIU guidelines (SIU; Grade A) state that DVIU or dilation is an appropriate initial procedure in bulbar urethral strictures of 2 cm or less. The AUA guidelines also mention that urethroplasty can be used for first time treatment of bulbar urethral strictures less than 2 cm (AUA; Grade C). For penile, penobulbar, multiple, and/or longer strictures, urethroplasty should be offered as initial management given the low success rate of endoscopic management (AUA; Grade C). SIU guidelines comment specifically that urethral reconstruction instead of DVIU or dilation is indicated for obliterative or near obliterative strictures (SIU; Grade A). In pediatric urethral strictures, DVIU or dilation is not recommended as a first line treatment given the high stricture recurrence rate (67–78% recurrence over 6 years) (16) (SIU; Grade A), and urethroplasty should be offered as the primary intervention.
According to the SIU guidelines, repeat DVIU or dilation is acceptable in favorable conditions such as solitary strictures, bulbar urethral strictures, length less than 2 cm, and stricture recurrence greater than 3 to 6 months after the previous treatment (SIU; Grade B). Recurrent stricture at 3 months or less after initial DVIU or dilation is predictive of poor success rate with second dilation and found to have no value after 48 months. On the other hand, if the stricture recurs more than 6 months after the initial procedure, repeat DVIU or dilation may produce a stricture free rate of 40% at 48 months (17). The AUA guidelines definitively state that urethroplasty is recommended over a second DVIU or dilation (AUA; Grade C). They do not make any stipulations on time of recurrence and therapy choice. More than one DVIU or dilation has proven to be cost ineffective compared to a definitive urethroplasty (SIU; Grade A). The SIU does state that a third DVIU or dilation should not be offered with the exception of situations limited by patient comorbidities or resource availability (SIU; Grade A). Repeat endoscopic procedures carry the risk of exacerbating spongiofibrosis and complicating definitive urethroplasty (SIU; Grade B). In fact, data suggests that prior endoscopic treatment for urethral stricture is an independent risk factor for stricture recurrence after urethroplasty (18).
In the setting of acute urinary retention, DVIU, urethral dilation, or suprapubic cystotomy is permitted as a temporizing intervention prior to urethroplasty (AUA; expert opinion). These can also be used as palliative measures combined with clean intermittent catheterization for patients who are unfit for major urethral reconstructive procedures (SIU; Grade B) (AUA; Grade C). After uncomplicated urethral dilation or DVIU, a catheter should be placed after the procedure and can be safely removed within 72 hours (AUA; Grade C).
Urethroplasty
Urethroplasty is the definitive procedure for urethral stricture disease. Urethroplasty has a success rate of up to 95% (19-21) with experienced surgeons. Surgeons who do not perform urethroplasty should refer patients to a surgeon with expertise in urethroplasty when indicated (AUA; expert opinion).
Bulbar strictures
For short strictures of the bulbar urethra, less than 2 cm, excision and primary anastomosis (EPA) urethroplasty is highly successful with a greater than 90% success rate for both primary and repeat procedures (SIU; Grade A). For longer strictures (2 to 4 cm), EPA is more likely to be successful if the stricture resides in the proximal bulb rather than the penile or distal bulbar urethra (SIU; Grade B). For strictures greater than 2 cm in the distal bulbar urethra or penile urethra, augmentation procedures should be utilized in place of EPA since a tension-free anastomosis may not be feasible (SIU; Grade B). Augmentation procedures may have lower success rates than EPA procedures, (SIU; Grade B) (22).
Substitution urethroplasty can be used to reconstruct longer strictures in a one stage or multistage approach using buccal mucosa or lingual oral grafts, penile fasciocutaneous flap, or a combination of both (AUA; Grade C). SIU recommends against tubularized graft urethroplasty (SIU; Grade A) and AUA specifies against single-stage tubularized graft urethroplasty (AUA; expert opinion). Grafts are preferred over flaps given greater mobility with grafts and similar success rates when compared to flaps (SIU; Grade B). Oral mucosa is the preferred choice for grafts in urethroplasty (AUA; expert opinion). Scrotal skin, which is associated with high morbidity (SIU; Grade A), and other hair-bearing skin should be avoided (AUA; clinical principal). Allografts, xenografts, or synthetic materials are not recommended outside of experiment protocols (AUA; expert opinion) (SIU; Grade B).
Meatal and fossa navicularis strictures
For meatal and fossa navicularis strictures, the AUA guidelines recommend initial management with dilation or meatotomy (AUA; clinical principal). Recurrent strictures of the meatus or fossa navicularis warrant urethroplasty (AUA; Grade C). In cases of hypospadias-related strictures, an urethroplasty is recommended (SIU; Grade B).
Penile urethral strictures
For penile strictures urethroplasty should be offered upfront (AUA; Grade C). In the case of substitution urethroplasty for penile strictures, onlay flaps should be considered (SIU; Grade B). If strictures are complex, procedures are usually more successful in two as opposed to single stage (SIU; Grade B). The AUA guidelines do not comment specifically on the success of staged procedures outside of the recommendation against single staged tubularized procedures (AUA; expert opinion). There are no definitive recommendations for catheter placement duration after urethroplasty or antibiotic duration after urethroplasty.
Vesicourethral stenosis, bladder neck contracture
Instances of bladder neck contracture as a complication of prostate procedures can be managed with dilation, bladder neck incision, or transurethral resection (AUA; expert opinion). In instances of post-prostatectomy vesicourethral stenosis, dilation, vesicourethral incision, or transurethral resection can be performed (AUA; Grade C) (SIU: Grade C). Open reconstruction is an option in cases where post-prostatectomy vesicourethral anastomotic stenosis is recalcitrant (AUA; Grade C).
Lichen sclerosus
In cases of suspected LS, clinicians may perform biopsy to confirm a diagnosis. In cases where cancer is suspected, biopsy must be performed (AUA; clinical principal). In cases were LS is in early stages and confined to the foreskin or glans, short-term topical steroids or circumcision should be considered (SIU: Grade A). For urethral strictures secondary to LS, genital skin should not be used when reconstruction is performed (AUA; Grade B). Instead, oral mucosa grafts are the preferred substitution material for LS-related strictures (23). Substitution urethroplasty utilizing bladder or colonic mucosa is discouraged (SIU; Grade C). Long-term follow-up is necessary for men and women with LS due to the increased potential for malignant transformation (SIU: Grade B).
Alternatives to urethroplasty
As an alternative to urethroplasty, the AUA guidelines support a perineal urethrostomy (AUA; Grade C). Permanent urethral stenting is not recommended in management of urethral strictures if patients are candidates for urethral reconstruction (SIU; Grade A). Permanent stenting may be considered in patients who cannot tolerate urethroplasty or intermittent self-dilation (SIU; Grade B). There are prostatic stents that, although backed by limited data, have been shown to have short-term success in brachytherapy patients (24) and patients with benign prostatic obstruction (25).
Post-operative follow up
For all procedures utilized to treat urethral strictures including DVIU, urethral dilation, or urethroplasty, it is important to monitor for stricture recurrence (AUA; expert opinion). Assessment with urethrography or urethroscopy can be used to determine recurrence (SIU; Grade A). Experts acknowledge that there is no gold standard for post-operative screening protocols but recommend a combination of patient symptom assessment, noninvasive test such as uroflowmetry, cystoscopy and/or RUG/VCUG (26,27).
Discussion/comments
When comparing the AUA and the SIU guidelines on urethral stricture, there are very few discrepancies. Perhaps the most notable differences are concern use of repeat DVIU or urethral dilation after an initial failed attempt. SIU guidelines state that there are instances where repeat DVIU or urethral dilation can be indicated, and they give a range of time at which stricture recurrence post procedure mandates an urethroplasty (less than 3 to 6 months). The AUA guidelines definitively state that repeat endoscopic procedures should not be offered as an alternative to urethroplasty, and they do not mention time of stricture recurrence as a factor.
Although few, the discrepancies between the recommendations offered by the two guidelines can be best explained by varying interpretations of the literature and available evidence on urethral strictures. It is important to note the difference in time of publication between the two guidelines. The AUA guidelines were published in 2016 and the SIU guidelines were published in 2010. Therefore, the cumulative body of evidence available at the time of publication of each guideline differs by six years. Newer studies substantiating effectiveness of urethroplasty relative to endoscopic procedures for urethral stricture (28,29) may explain the more current AUA guidelines recommendation against repeat DVIU/dilations. However, the lack of definitive, high quality evidence to support a substantial portion of the guidelines hinders the formulation of unvarying and indisputable best practice recommendations.
The AUA guidelines specify a need for standardized research terms to allow for comparison between centers, multiple criteria for treatment success, improved duration and follow up criteria, and multi-institutional collaboration. They make recommendations for further research into etiology of urethral strictures, improved and unified educational efforts for patients and health care practitioners, and consensus treatment of LS. In addition, they point out knowledge gaps in autologous graft material, anti-proliferative injections, erectile dysfunction, prophylactic antibiotics, and ideal tissue determination for substitution urethroplasty.
Conclusions
Overall there is a need for more high quality research in the work up, management, and follow up care of urethral stricture. There is very few large scale, multi-institutional studies leading to consensus high-grade recommendations. Continually improving evidence quality will yield future guidelines with more robustly supported and aligned recommendations.
Acknowledgements
This work was supported by the Alafi Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Latini JM, McAninch JW, Brandes SB, et al. SIU/ICUD Consultation On Urethral Strictures: Epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology 2014;83:S1-7. [Crossref] [PubMed]
- Lumen N, Hoebeke P, Willemsen P, et al. Etiology of urethral stricture disease in the 21st century. J Urol 2009;182:983-7. [Crossref] [PubMed]
- Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol 2007;177:1667-74. [Crossref] [PubMed]
- Mundy AR. Words of wisdom. Re: Outcome of dorsal buccal graft urethroplasty for recurrent urethral strictures. Eur Urol 2009;55:991-2. [Crossref] [PubMed]
- Wessells H, Angermeier KW, Elliott SP, et al. Male Urethral Stricture. American Urological Association (AUA) Guideline 2016:1-34.
- Jordan G, Chapple C, Heyns C. Urethral Strictures. Presented at An International Consultation on Urethral Strictures, Marrakech, Morocco, October 13-16, 2010.
- Bryk DJ, Zhao LC. Guideline of guidelines: a review of urological trauma guidelines. BJU Int 2016;117:226-34. [Crossref] [PubMed]
- Stein DM, Thum DJ, Barbagli G, et al. A geographic analysis of male urethral stricture aetiology and location. BJU Int 2013;112:830-4. [Crossref] [PubMed]
- Erickson BA, Breyer BN, McAninch JW. Changes in uroflowmetry maximum flow rates after urethral reconstructive surgery as a means to predict for stricture recurrence. J Urol 2011;186:1934-7. [Crossref] [PubMed]
- Angermeier KW, Rourke KF, Dubey D, et al. SIU/ICUD Consultation on Urethral Strictures: Evaluation and follow-up. Urology 2014;83:S8-17. [Crossref] [PubMed]
- Bach P, Rourke K. Independently interpreted retrograde urethrography does not accurately diagnose and stage anterior urethral stricture: the importance of urologist-performed urethrography. Urology 2014;83:1190-3. [Crossref] [PubMed]
- Goonesinghe SK, Hillary CJ, Nicholson TR, et al. Flexible cystourethroscopy in the follow-up of posturethroplasty patients and characterisation of recurrences. Eur Urol 2015;68:523-9. [Crossref] [PubMed]
- Heidenreich A, Derschum W, Bonfig R, et al. Ultrasound in the evaluation of urethral stricture disease: a prospective study in 175 patients. Br J Urol 1994;74:93-8. [Crossref] [PubMed]
- Steenkamp JW, Heyns CF, de Kock ML. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J Urol 1997;157:98-101. [Crossref] [PubMed]
- Roosen JU. Self-catheterization after urethrotomy. Prevention of urethral stricture recurrence using clean intermittent self-catheterization. Urol Int 1993;50:90-2. [Crossref] [PubMed]
- Launonen E, Sairanen J, Ruutu M, et al. Role of visual internal urethrotomy in pediatric urethral strictures. J Pediatr Urol 2014;10:545-9. [Crossref] [PubMed]
- Heyns CF, Steenkamp JW, De Kock ML, et al. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J Urol 1998;160:356-8. [Crossref] [PubMed]
- Breyer BN, McAninch JW, Whitson JM, et al. Multivariate analysis of risk factors for long-term urethroplasty outcome. J Urol 2010;183:613-7. [Crossref] [PubMed]
- Barbagli G, Kulkarni SB, Fossati N, et al. Long-term followup and deterioration rate of anterior substitution urethroplasty. J Urol 2014;192:808-13. [Crossref] [PubMed]
- Cooperberg MR, McAninch JW, Alsikafi NF, et al. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol 2007;178:2006-10; discussion 2010.
- Barbagli G, De Angelis M, Romano G, et al. Long-term followup of bulbar end-to-end anastomosis: a retrospective analysis of 153 patients in a single center experience. J Urol 2007;178:2470-3. [Crossref] [PubMed]
- Kluth LA, Dahlem R, Reiss P, et al. Short-term outcome and morbidity of different contemporary urethroplasty techniques--a preliminary comparison. J Endourol 2013;27:925-9. [Crossref] [PubMed]
- Levine LA, Strom KH, Lux MM. Buccal mucosa graft urethroplasty for anterior urethral stricture repair: evaluation of the impact of stricture location and lichen sclerosus on surgical outcome. J Urol 2007;178:2011-5. [Crossref] [PubMed]
- de Graaf GW, Stijns PE, Scheepens WA, et al. The use of a memokath prostatic stent for obstructive voiding symptoms after brachytherapy. Curr Urol 2013;7:19-23. [Crossref] [PubMed]
- Yildiz G, Bahouth Z, Halachmi S, et al. Allium™ TPS--A New Prostatic Stent for the Treatment of Patients with Benign Prostatic Obstruction: The First Report. J Endourol 2016;30:319-22. [Crossref] [PubMed]
- Erickson BA, Breyer BN, McAninch JW. The use of uroflowmetry to diagnose recurrent stricture after urethral reconstructive surgery. J Urol 2010;184:1386-90. [Crossref] [PubMed]
- Zaid UB, Hawkins M, Wilson L, et al. The cost of surveillance after urethroplasty. Urology 2015;85:1195-9. [Crossref] [PubMed]
- Al Taweel W, Seyam R. Visual Internal Urethrotomy for Adult Male Urethral Stricture Has Poor Long-Term Results. Adv Urol 2015;2015:656459. [Crossref] [PubMed]
- Anger JT, Buckley JC, Santucci RA, et al. Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty? Urology 2011;77:481-5. [Crossref] [PubMed]


