Testosterone deficiency in adults and corresponding treatment patterns across the globe
Introduction
Driven by an increasingly aging population, the treatment of testosterone deficiency (TD) is experiencing a more frequent focus in sexual medicine. The prevalence of TD varies between 10–40% (1-4), and is known to increase with age, with an abrupt rise in men aged 45–50 years (4). Specifically, TD coincides with the presence of comorbidities, reaching as high as 80% in patients with multiple comorbidities (5-9). Although highly prevalent, the symptoms of TD are often successfully managed with testosterone replacement therapy (TRT), making the accurate diagnosis and appropriate treatment of TD an important topic of clinical interest.
TD is most commonly seen as a component of male hypogonadism (HG), which is a clinical syndrome related to the inadequate production of testosterone (androgen deficiency) and reduced spermatogenesis. Male HG represents biochemical HG and/or symptomatic androgen deficiency. Biochemical HG is defined as a serum total testosterone (TT) level below a pre-determined threshold value, with several variations existing in the literature, usually between 200 and 400 ng/dL (10-12). Unfortunately, there is no commonly agreed upon and universally used TT-calibration laboratory standard, introducing added variability when defining a TT cut-off (13). The prevalence of biochemical HG increases with age, ranging from 12% among men in their 50s to 49% among those 80 years and older (14). Symptomatic androgen deficiency, on the other hand, is the presence of at least three signs or symptoms consistent with androgen deficiency and confirmed by a serum TT or free testosterone (FT) below the normal cut-off value. Symptomatic androgen deficiency is significantly less prevalent (6% only) than biochemical HG (15). Symptoms of androgen deficiency are often nonspecific and include diminished libido and erectile dysfunction, weight gain, fatigue, poor concentration and depressed mood (6,10). There are significant differences in practice patterns regarding the diagnosis of HG, with some physicians placing more emphasis on biochemical HG, others on patient-reported symptoms, and most settling on a combination of the two. For example, it has been previously recommended by the International Society for Sexual Medicine (ISSM) that those with TT <200 ng/dL definitely have HG and should be treated; those with TT >400 definitely do not have TD; and those with TT between 200 and 400 should be treated based on their clinical symptoms, if they are symptomatic (12). No universal consensus has been reached, making the diagnosis and treatment of HG a controversial topic.
Fortunately, TRT has been shown to improve the symptoms of HG, as well as overall quality of life (QOL) (16-24), albeit at the cost of a low but potentially significant risk of adverse events (AEs). An increase in serum hematocrit is the most common AE (25), which in extreme cases can lead to serum hyperviscosity, which has been associated with vascular thromboembolic events such as stroke, myocardial infarction, and deep vein thrombosis.
Although it is clear that the clinical burden of TD is significant, and its treatment has the potential to improve QOL, many questions remain surrounding testosterone prescribing patterns around the world. This article discusses the incidence and prevalence of primary TD and male HG and the corresponding patterns of TRT across the globe. The clinical manifestations and laboratory diagnosis of male HG, and the evaluation and management of secondary male HG are not the subject of this review.
Global prevalence of TD and primary HG
Although the measurement of serum testosterone levels is commonly used in the diagnosis of male HG, the cut-off values for low testosterone are not well defined and vary throughout the literature, ranging from 200–400 ng/dL, with 300 ng/dL being the most commonly utilized in clinical practice. Other measurements such as FT may also be used. Although cut-off is an important component in diagnosing HG, guidelines from the United States Endocrine Society define HG as symptoms of HG with TT <300 ng/dL, which differs from the cut-off of 249 ng/dL recommended by the European Association for Urology (10,26).
A summary of the global prevalence of TD and HG can be seen in Table 1. The prevalence of TD in middle-aged to elderly men in the United States ranged from 24–39%—a prevalence much higher than that observed in other parts of the world (4,15,27,28). This prevalence dropped to 6% when Araujo et al. evaluated HG by applying the combination of a TT threshold of 200 ng/dL and the presence of three patient-reported symptoms of HG (15), demonstrating that there is a significant difference between biochemical and clinical HG. The prevalence of diagnosed HG in Europe was significantly lower than in the US, ranging from 8–20% (9,29-31), but again appeared to be significantly lower when only diagnosed in patients with both low testosterone as well as symptoms of androgen deficiency (32). Studies in Asia and South America demonstrated a similar trend, with the prevalence of biochemical HG ranging from 17–33%, but dropping to just half of that (10–12%) when requiring the presence of relevant symptoms (3,9,33-35). HG data is lacking in Middle-Eastern populations, but studies from Jordan and Saudi Arabia (S-Arabia) showed a prevalence of 8–24% (29,36). Prevalence data in Africa was scarce, and the literature was clouded by the existence of comorbid diseases such as diabetes mellitus. Overall, although the United States seems to have a higher prevalence of diagnosed HG, prevalence rates are likely similar to other geographic locations.
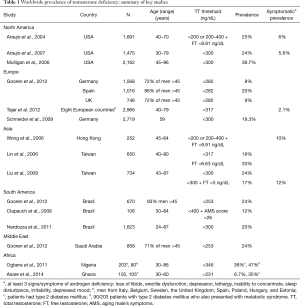
Full table
It is clear that the major problem in estimating the prevalence of HG is the inconsistent data on what physicians emphasize most during diagnosis. Manifestations of male HG are often nonspecific and subclinical, and are influenced by several factors including patient’s age and presence of comorbidities. The diagnosis of male HG is further complicated by significant variations in the laboratory tests for TT. This conflicting data leads to significant differences in reported prevalence and prescribing patterns.
Testosterone prescribing patterns
While many similarities exist in testosterone prescribing patterns, there are also important geographic differences in the frequency of laboratory testing and use of clinical symptoms to diagnose and treat TD and HG. This often leads to significant differences in the prevalence of testosterone prescriptions. A closer look at the literature available for each part of the world (Table 2) can shed light on these pertinent similarities and differences.
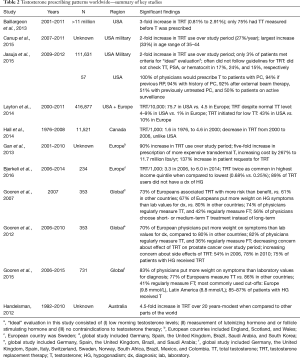
Full table
North America
The United States has the reputation of being one of the heaviest prescribers of testosterone (37). In a study of over ten million Americans (38), testosterone use in men older than 40 was shown to have increased by more than 3-fold between the years 2001 and 2011. While often prescribed, many patients do not have laboratory-documented TD. According to this study, the most predominant reasons for testosterone prescription were HG (51%), fatigue (35%), erectile dysfunction (32%), and psychosexual dysfunction (12%). One-fifth of new users received treatment for less than 30 days, and only three-fourths had their testosterone level measured in the prior year. Moreover, it was unclear how many of those men actually had abnormal results, leading the authors to conclude that the reasons for initiating TRT appear to be complex.
A study of prescribing patterns in United States military treatment facilities showed a more than 2-fold increase in testosterone prescriptions between 2007 and 2011 (39). Another study examined testosterone prescription pattern in a military population within the Veterans Affairs system between 2009 and 2012 (40). Interestingly, only 3% of men who received TRT met criteria for an “ideal” evaluation, consisting of (I) low morning testosterone levels, (II) measurement of luteinizing hormone and or follicle stimulating hormone, and (III) no contraindications to TRT. In addition, physicians did not routinely obtain follow-up serum TT, prostate specific antigen, or hematocrit, contrary to TRT guidelines.
A controversial topic in the TRT debate is whether or not patients with HG should receive TRT if they also have a history of prostate cancer. A 2015 electronic survey of North American members of the Sexual Medicine Society of North America (SMSNA) revealed that a current or previous diagnosis of prostate cancer did not hinder physicians from prescribing TRT (41). These findings contrasted with a similarly designed 2015 German study which showed that German urologists were far less likely than their American counterparts to prescribe TRT to patients with a history of prostate cancer (42).
In a study comparing TRT in the United States to that of the United Kingdom (UK) between 2000 and 2011 (37), it was observed that while TRT increased in both countries, the increase was pronouncedly higher in the United States. Notably, the United States prescribers tended to initiate TRT at normal TT levels, or even without checking levels, more often than those in the UK. In addition, American men who were tested had more comorbidities (hypertension, diabetes, and cardiovascular disease) than those tested in the UK. One of the main reasons for TT testing in the United States included fatigue, which was a very infrequent practice in the UK. The authors attributed most of these differences to heavy direct-to-consumer marketing of new testosterone formulations in the United States, leading to more treatment in men with non-specific symptoms, as opposed to targeted testing of symptomatic individuals.
In Canada, studies have similarly shown an increase in TRT, although to a lesser extent than in the United States. A study of the Canadian health care system over 12 years (43) found that the annual TRT prescription rate increased only modestly, from 1.6/1,000 in 1976 to 4.6/1,000 in 2000, with substantial variation over time. Contrary to most global studies on TRT prescribing patterns, there was an annual decrease between 2000 and 2006, thought to be due to the substitution of androgens with newly available oral treatments for erectile dysfunction, namely phosphodiesterase type 5 inhibitors (PDE5-I), which became available in Canada in 1999. A subsequent increase in TRT was noted in 2008. In another Canadian study, nine men who had used TRT, and 13 providers, were interviewed for a qualitative analysis of therapy (44). The factors that were found to influence testosterone prescription for providers included concerns about diagnostic ambiguity of age-related HG (which tests to use, what is the adequate threshold value for HG, etc.) and the appropriateness of TRT. Patient factors included access to information about testosterone therapy. The study concluded that much work is needed to improve diagnostic accuracy, and that both providers and patients need more information about the risks and benefits of TRT.
Europe
While not as prevalent as in the United States, testosterone use in Europe has been studied extensively. Prescriptions for TRT increased by nearly 90% over a 10-year period (2001 to 2010) in a study in England, Scotland, and Wales (30). The authors noted that while the prevalence of diagnosed primary HG, cancer-survivorship, and opioid use were on the rise and may have contributed to the increase in TRT, it was unlikely that these were the only reasons. A local survey performed by the same group revealed that the absolute number of men with unequivocal HG has remained relatively constant, suggesting that many eugonadal men may be receiving unnecessary treatment (45).
Similar trends have been observed in other European countries (46). The rate of TRT prescription almost doubled, according to a Swedish study, from 2006–2014. This increase was particularly notable among men in the highest income quintile when compared to those in the lowest. The highest rate of TRT was in divorced men and those aged 65–69 years. Interestingly, an overwhelming majority of men who received TRT did not have a diagnosis of HG, which the authors attributed to an increased interest in using testosterone as a means to retain virility in cases of age-related reduction in testosterone production.
Overall, it appears that there was a dramatic rise in the rates of TT testing and TRT in the United States when compared to Canada and other European countries during the same time period.
The differences between countries in Europe and those in Africa, Asia, and South America have also been examined in several studies. In a global survey of physicians conducted in 2007, researchers inquired about the reasons for and against the use of TRT (47). The fear of inducing prostate cancer was a very powerful motivator against TRT, but more so in Europe than elsewhere. Most physicians associated TRT with a greater risk than benefit, leading to over one-third of patients not receiving treatment. Most physicians were motivated by symptoms, rather than by laboratory results (but less so than physicians in the other countries analyzed in the study). There was not much difference between Europe and the rest of the world in measuring TT, but luteinizing hormone was more regularly measured in Europe (63%) than elsewhere. The inconsistent use of biochemical means for the diagnosis of HG, along with differing emphasis on clinical symptoms, invoked a high amount of variability in prescribing patterns.
A global study conducted by the same group between 2006 to 2010 attempted to identify the most influential factors for initiating TRT (29). Similar to the previous study, European physicians were much more likely than their counterparts to consider the severity of HG-related complaints rather than measured serum TT levels in their decision to provide TRT. Those who placed more emphasis on symptoms of androgen deficiency ranged from 7–96%, highlighting practice variability. Over time, physicians were more likely to provide TRT and less likely to express concerns about prostate cancer in 2010 when compared to 2006, but more likely to be concerned about other side effects. The main reasons for not giving therapy to older patients (>45 years old) were clinicians’ concerns about prostate cancer and patients’ own concerns about treatment. For younger patients, it was attributed to alternative treatments (sports and diet), which were recommended more often in 2010 than in 2006.
To further evaluate these trends from 2006 to 2015, the same authors interviewed 731 physicians worldwide (48). When compared to their previous studies, more emphasis was placed on clinical symptoms of HG than laboratory values of TT. European physicians were less inclined to measure TT than their Latin American counterparts. Interestingly, ~10% of participating physicians did not know the cut-off level for TT in the diagnosis of HG, and less than half used a certain value of TT for diagnosis, again demonstrating the inconsistency in diagnosing HG. European practices had the least stringent cut-off for the diagnosis of HG, but all thresholds used were lower than the cut-off of 300 ng/dL cited the most in the literature (49). Once diagnosed with HG, the majority of patients received testosterone prescriptions, but treatment compliance was problematic with 36% of patients deciding not to start or continue treatment.
South America
Although less literature is available on TRT in South America, trends from the region could be garnered from global studies. After interviewing 50 Brazilian physicians, it was noted that Brazil had a higher incidence of diagnosed TD (10%) when compared to Europe (5–8%) (47). Brazilian physicians were more inclined to continue treating patients who had serious concerns about treatment, and were more receptive to evidence demonstrating that TRT is safe in the setting of a diagnosis of prostate cancer.
Another world study on trends of testosterone use between 2006 and 2010 included Brazil (29). Similar to the previous study, TD was highest in Brazil, but even more notably so, reaching 24% during this time period, which starkly contrasted with rates seen in the UK and Germany (8–9%). Brazilian physicians placed more weight on the symptoms of TD, rather than on serum TT values. Improvement in sexuality was the predominant reason to initiate treatment in Brazil, while improvement of libido was the leading cause in Europe and S-Arabia.
Global testosterone prescribing trends between 2007 and 2015 were recently examined (48). Latin American physicians obtained testosterone laboratory values more often than Europeans (86% vs. 77%) and 87% indicated that the severity of HG symptoms carried greater weight than the laboratory values when deciding to treat, in keeping with previous reports (29). Once a diagnosis of HG was obtained, Latin American physicians initiated treatment in 85–87% of patients, which was higher than rates recorded from 2006–2010 (63–74%).
Australia
Testosterone treatment patterns have been studied in Australia since the early 1990’s. In a study looking at the expenditure on TRT in Australia between 1991 and 2001, there were two significant upsurges (1993–1994 and 1998–1999), followed by declines, in national prescribing of testosterone (50). The increase in TRT prescription revenue during the 1990s in Australia was modest, which sharply contrasted with a 20-fold increase in revenue from TRT sales in the US during the same time period.
In another study evaluating almost 20 years of prescribing TRT in Australia [1992–2010], a 4.5 increase in total annual expenditures on TRT products were described (51). The researchers postulated that the overall increase in testosterone usage could not be explained solely by an increased diagnosis of HG, and attributed it to robust marketing and advertising for indications such as “andropause” and sexual dysfunction.
Asia, Africa, and the Middle East
The data on testosterone-prescribing patterns in Asia is scarce. Fifty South Korean physicians were surveyed in a 2007 global study (47). When compared to physicians elsewhere, South Korean physicians were the least concerned about prostate cancer when prescribing TRT, with only 2% reporting it was a factor in their decision to avoid treatment. Oral TRT was more frequently used in South Korea (33%) when compared to most other countries (8–9%). Lastly, it was noted that South Korean physicians were the least likely to measure testosterone levels in patients with sexual complaints, with only 26% of physicians doing so (compared to 84% in S-Arabia). More studies are needed to better elucidate testosterone-prescribing patterns in Asia.
A lack of literature on TRT patterns in Africa necessitates the extrapolation from global studies. In an international study of testosterone use between 2006 and 2015, surveys from 262 testosterone prescribers in South Africa were analyzed (48). It was found that the greatest limiting factor in patient compliance in South Africa was the financial burden of long-term treatment, commented by up to 79% of physicians, significantly higher than elsewhere. Problems with reimbursement were also especially prominent in South Africa. Like Asia, more studies are needed to elucidate testosterone-prescribing patterns in Africa.
The literature on TRT in the Middle East is similarly limited. In the 2007 global study, 63 physicians from S-Arabia were surveyed (47) and found most likely to increase testosterone prescription if it could be proven that this medication was not dangerous in patients with prostate cancer. Oral treatment for testosterone was much more commonly used in S-Arabia (46%) when compared to Europe (8–9%). Lastly, 84% of Saudi Arabian physicians measured testosterone levels when patients presented with ED, significantly higher than the 63% proportion observed in the rest of the world. In the second global study [2006–2010] (29), Saudi physicians reported less influence of clinical symptoms as a reason to start TRT when compared to physicians from other countries (36% vs. 70%). Popular reasons to start therapy in S-Arabia include low testosterone levels and hope for improvement in erectile function.
Conclusions
Most of the available data on TRT are derived from North America and Europe, but an estimate of the trends in other continents can be made from global studies. A significant limitation of the published data is the inconsistent methodology used to identify HG, namely differing emphasis placed on biochemical HG vs. symptomatic androgen deficiency. While there are significant differences in factors used to prescribe testosterone, most countries appear to follow the same strategy. More investigation is needed to better describe these various patterns, especially in Asia, Africa, and the Middle East.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Khoo EM, Tan HM, Low WY. Erectile dysfunction and comorbidities in aging men: an urban cross-sectional study in Malaysia. J Sex Med 2008;5:2925-34. [Crossref] [PubMed]
- Ponholzer A, Madersbacher S, Rauchenwald M, et al. Vascular risk factors and their association to serum androgen levels in a population-based cohort of 75-year-old men over 5 years: results of the VITA study. World J Urol 2010;28:209-14. [Crossref] [PubMed]
- Wong SY, Chan DC, Hong A, et al. Prevalence of and risk factors for androgen deficiency in middle-aged men in Hong Kong. Metabolism 2006;55:1488-94. [Crossref] [PubMed]
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762-9. [Crossref] [PubMed]
- Pellitero S, Olaizola I, Alastrue A, et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes Surg 2012;22:1835-42. [Crossref] [PubMed]
- Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 2011;34:1669-75. [Crossref] [PubMed]
- Atlantis E, Martin SA, Haren MT, et al. Demographic, physical and lifestyle factors associated with androgen status: the Florey Adelaide Male Ageing Study (FAMAS). Clin Endocrinol (Oxf) 2009;71:261-72. [Crossref] [PubMed]
- Haring R, Ittermann T, Volzke H, et al. Prevalence, incidence and risk factors of testosterone deficiency in a population-based cohort of men: results from the study of health in Pomerania. Aging Male 2010;13:247-57. [Crossref] [PubMed]
- Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 2008;93:2737-45. [Crossref] [PubMed]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536-59. [Crossref] [PubMed]
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol 2008;159:507-14. [Crossref] [PubMed]
- Hellstrom WJ, Paduch D, Donatucci CF. Importance of hypogonadism and testosterone replacement therapy in current urologic practice: a review. Int Urol Nephrol 2012;44:61-70. [Crossref] [PubMed]
- Rosner W, Auchus RJ, Azziz R, et al. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 2007;92:405-13. [Crossref] [PubMed]
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724-31. [Crossref] [PubMed]
- Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004;89:5920-6. [Crossref] [PubMed]
- Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381-94. [Crossref] [PubMed]
- Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004;89:2085-98. [Crossref] [PubMed]
- Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 2000;85:2670-7. [PubMed]
- Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes 2013;3:73-83. [Crossref] [PubMed]
- Francomano D, Ilacqua A, Bruzziches R, et al. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology 2014;83:167-73. [Crossref] [PubMed]
- Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53. [PubMed]
- Wang C, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab 1996;81:3578-83. [PubMed]
- Miner MM, Bhattacharya RK, Blick G, et al. 12-month observation of testosterone replacement effectiveness in a general population of men. Postgrad Med 2013;125:8-18. [Crossref] [PubMed]
- Pope HG Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11. [Crossref] [PubMed]
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010;95:2560-75. [Crossref] [PubMed]
- Dohle GR, Colpi GM, Hargreave TB, et al. EAU guidelines on male infertility. Eur Urol 2005;48:703-11. [Crossref] [PubMed]
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241-7. [Crossref] [PubMed]
- Hall SA, Esche GR, Araujo AB, et al. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab 2008;93:3870-7. [Crossref] [PubMed]
- Gooren LJ, Behre HM. Diagnosing and treating testosterone deficiency in different parts of the world: changes between 2006 and 2010. Aging Male 2012;15:22-7. [Crossref] [PubMed]
- Gan EH, Pattman S, H S, Pearce S, et al. A UK epidemic of testosterone prescribing, 2001-2010. Clin Endocrinol (Oxf) 2013;79:564-70. [Crossref] [PubMed]
- Schneider HJ, Sievers C, Klotsche J, et al. Prevalence of low male testosterone levels in primary care in Germany: cross-sectional results from the DETECT study. Clin Endocrinol (Oxf) 2009;70:446-54. [Crossref] [PubMed]
- Tajar A, Huhtaniemi IT, O'Neill TW, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab 2012;97:1508-16. [Crossref] [PubMed]
- Lin YC, Hwang TI, Chiang HS, et al. Correlations of androgen deficiency with clinical symptoms in Taiwanese males. Int J Impot Res 2006;18:343-7. [Crossref] [PubMed]
- Li JY, Li XY, Li M, et al. Decline of serum levels of free testosterone in aging healthy Chinese men. Aging Male 2005;8:203-6. [Crossref] [PubMed]
- Liu CC, Wu WJ, Lee YC, et al. The prevalence of and risk factors for androgen deficiency in aging Taiwanese men. J Sex Med 2009;6:936-46. [Crossref] [PubMed]
- Al Hayek AA, Khawaja NM, Khader YS, et al. The prevalence of Hypogonadism among diabetic and non-diabetic men in Jordan. J Diabetes Complications 2014;28:135-40. [Crossref] [PubMed]
- Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab 2014;99:835-42. [Crossref] [PubMed]
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. [Crossref] [PubMed]
- Canup R, Bogenberger K, Attipoe S, et al. Trends in Androgen Prescriptions From Military Treatment Facilities: 2007 to 2011. Mil Med 2015;180:728-31. [Crossref] [PubMed]
- Jasuja GK, Bhasin S, Reisman JI, et al. Ascertainment of Testosterone Prescribing Practices in the VA. Med Care 2015;53:746-52. [Crossref] [PubMed]
- Contemporary prescribing patterns of testosterone replacement therapy in hypogonadal patients with a history of prostate cancer. Available online: https://www.auanet.org/university/abstract_detail.cfm?id=MP51-14&meetingID=15NOLA
- Kühn CM, Strasser H, Romming A, et al. Testosterone Replacement Therapy in Hypogonadal Men Following Prostate Cancer Treatment: A Questionnaire-Based Retrospective Study among Urologists in Bavaria, Germany. Urol Int 2015;95:153-9. [Crossref] [PubMed]
- Hall SA, Ranganathan G, Tinsley LJ, et al. Population-based patterns of prescription androgen use, 1976-2008. Pharmacoepidemiol Drug Saf 2014;23:498-506. [Crossref] [PubMed]
- Mascarenhas A, Khan S, Sayal R, et al. Factors that may be influencing the rise in prescription testosterone replacement therapy in adult men: a qualitative study. Aging Male 2016;19:90-5. [Crossref] [PubMed]
- Gan EH, Pattman S, Pearce S, et al. Many men are receiving unnecessary testosterone prescriptions. Bmj 2012;345:e5469. [Crossref] [PubMed]
- Bjerkeli PJ, Mulinari S, Merlo J. Testosterone prescribing in the population-a short social epidemiological analysis in Sweden. Pharmacoepidemiol Drug Saf 2016;25:11-5. [Crossref] [PubMed]
- Gooren LJ, Behre HM, Saad F, et al. Diagnosing and treating testosterone deficiency in different parts of the world. Results from global market research. Aging Male 2007;10:173-81. [Crossref] [PubMed]
- Gooren L. Diagnosing hypogonadism and treating decisions in different parts of the world: shifts in patterns between 2006 and 2015. Aging Male 2016;19:46-53. [Crossref] [PubMed]
- Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab 2011;96:2430-9. [Crossref] [PubMed]
- Handelsman DJ. Trends and regional differences in testosterone prescribing in Australia, 1991-2001. Med J Aust 2004;181:419-22. [PubMed]
- Handelsman DJ. Pharmacoepidemiology of testosterone prescribing in Australia, 1992-2010. Med J Aust 2012;196:642-5. [Crossref] [PubMed]


