Penile prosthesis implant: scientific advances and technological innovations over the last four decades
Introduction
Scientific advances in our understanding of penile erectile mechanism and subsequent development of effective therapy in erectile dysfunction (ED), have revolutionized the management for ED. Three sentinel events in ED treatment that have occurred in the last four decades were the development of inflatable penile prosthesis (IPP) in 1973, which allowed for the possibility of rigid (artificial) penile erection (1), followed by a decade later with the introduction of various intracavernous vasoactive agents such as phentolamine and papaverine, allowing for the first time a truly effective medical treatment for many men (2,3). In the late 1990s, the advent of oral phosphodiesterase type 5 inhibitor, sildenafil citrate became the first effective oral therapy for ED, and acknowledged as the first line standard of care for men with ED (4).
Despite introduction of oral phosphodiesterase type 5 inhibitors and intracavernosal vasoactive agents, penile prosthesis implant remains a relevant and desired option as many men became refractory to medical therapy and/or seeking a more effective and permanent therapy. The first detailed attempt at implanting an artificial device in the penis to correct ED was performed in 1936 by Bogaras, who reconstructed an amputated penis using an abdominal tube pedicle graft (5). The concept introduced by Bogaras was further improved upon by others with the insertion of a section of rib cartilage into the reconstructed penis to provide better penile rigidity (6,7). Goodwin and Scott (8) were the first to utilize acrylic stents in penile reconstructive surgery in the early 1950s, while Beheri (9) described the use of a paired intracorporeal polyethylene rods in 1960s to treat ED. Around the same time, Lash (10,11) and Pearman (12) reported the use of single, silicone rods implanted under the fascia of the penile shaft, to provide stability and “erection” in the penis. A few years later, Pearman revised the insertion of his prosthesis to beneath the tunica albuginea for better concealment and “feel” to the penis (13). However, these early penile prostheses had high complication rates from mechanical issues and prosthesis erosion, resulting in poor acceptance and were subsequently discontinued.
The following article reviews the scientific advances and technological innovation in modern penile prosthesis implants over the last four decades.
Methods
A comprehensive review of all relevant publications pertaining to penile prosthesis design and technology over the last four decades was performed. Matters related to patient selection and complications related to penile prosthesis implant were excluded in this review.
Types of penile prosthesis implants
There are two types of penile prosthesis implants: inflatable and non-inflatable types, and the inflatable penile implants can be subdivided into single-, two- and three-piece devices (see Table 1).
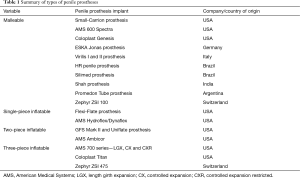
Full table
Non-inflatable (malleable) penile prosthesis implants
Non-IPP may be referred to as semi-rigid rod or malleable prosthesis. This device usually consists of a pair of rods made of either spiral wire core or silicone material, wrapped in fabric such as silicone or polyurethane jacket. Small and Carrion popularized the use of semi-rigid penile prosthesis implant (Small-Carrion prosthesis), a paired sponge-filled semi-rigid silicone implants (14). The introduction of the Small-Carrion prosthesis (Mentor, Minneapolis, MN, USA) in 1975 helped pave the wave for a new era of malleable penile prostheses (15).
A variety of semi-rigid prostheses are currently commercially available worldwide and two of the largest and most commercially successful malleable prosthesis implants are the American Medical Systems (AMS) 600 Spectra (Minnetonka, MN, USA) and Coloplast Genesis (Minneapolis, MN, USA). The Boston Scientific (Marlborough, Massachusetts, USA) which has recently acquired AMS, owned the AMS malleable 600 Spectra prosthesis which has articulated segments (ball and socket joints) of polyethylene material that is held together by a central spring, providing positional memory and allowing the malleable rods to remain concealed when not in use, yet sufficiently rigid for penetrative intercourse (16). The Mentor (Coloplast) malleable and the Acu-Form penile prosthesis were produced initially by Mentor Corporation (Minneapolis, MN, USA), and now the Coloplast Corporation (17). In 2004, Coloplast introduced the Genesis one-piece malleable device with a hydrophilic coating that allows the surgeon to maintain the device preparation with their own choice of antibiotics, and device is trimmable with the use of rear tip extenders to add to prosthesis length (18).
Other examples of semi-rigid prostheses manufactured across the world are the Flexi-Rods (USA), ESKA Jonas prosthesis (Germany), Virilis I and II implants (Italy), Silimed penile prosthesis (Brazil), HR penile prosthesis (Brazil), Shah penile implant (India), Promedon Tube prosthesis (Argentina) and Zephyr ZSI 100 (Switzerland), with most of these prostheses sales largely confined to selected countries with limited commercial success and some having withdrawn from the market. In 1997, Finney introduced the Flexi-Rod prosthesis (Surgitek, Racine, WI, USA), a paired semi-rigid implant with a softer proximal portion to provide better concealment and a trimmable tail to reduce inventory, an improvement to the Small-Carrion malleable prosthesis (19). The silicone core was later reinforced with Dacron to create a firmer prosthesis, and renamed the Flexi-Rod II. In 1980, Jonas and Jacobi developed the first German malleable device consisting of paired silicone implant with a twisted silver wire core to increase rigidity and can be bent either downward or upward (20). This German implant, the ESKA Jonas prosthesis (C. R. Bard, Covington, GA, USA) underwent further enhancement with Teflon coating to the silver wire core to improve mechanical durability (21). The Virilis I implant (Giant Medical, Cremona, Italy) from Italy is made with soft medical grade silicone while the Virilis II has a firmer distal portion. Both soft implants displace the erectile tissue without cavernosal tissue destruction and allow for more natural penile tissue distension due to preservation of underlying cavernous blood flow. Another Italian penile device, the Apollo implant (Giant Medical, Cremona, Italy) is a temporary penile prosthesis designed to produce tissue length expansion through periodic injections of normal saline into the distal portion of the device so that greater penile length can be been obtained postoperative. The two Brazilian malleable implants are the HR penile prosthesis, consisting of two malleable rods where one with a steel core and the second with a silver cord (similar to the Jonas model) while the Silimed malleable implant (Silimed, Rio de Janeiro, RJ, Brazil) is composed of silicone elastomer with a silver core and adjustable rear tip for length expansion. The Argentinean Promedon Tube prosthesis (Cesar Ortiz Promedon, Argentina) is a malleable silicone implant with poly-tetra-fluoro-ethylene-coated silver core and has a trimmable proximal segment (22). The Shah implant from India is a silicone nonmalleable device with four zones of stiffness—a soft distal tip followed by a stiff segment for shaft rigidity, next a soft zone that can hinge, and finally a narrow stiff proximal zone (23). The most recent addition to the market is the ZSI 100 malleable penile prosthesis (Zephyr Surgical Implants SRAL, Geneva, Switzerland) which comes in either in standard or extra rigid prosthesis size.
Dacomed popularized the idea of a “positionable” penile prosthesis which allows for central tensioning of the internal series articulating plastic (polysulfone) segments, rendering previously flaccid prosthesis “positionable”. The OmniPhase prosthesis (Dacomed, Minneapolis, MN, USA) has a mechanism which alters the length of the central cable, resulting in tensioned (erect) and flaccid prosthesis but its central cable often break down (24). This device was later revised as DuraPhase prosthesis where a central tensioning cable which ran through 12 articulating polysulfone segments is connected at both ends to springs secured metal housings but cable breakage remains a problem (25).
IPP
The IPP was developed to stimulate normal penile erection, and they consist of a pair of cylinders implanted in the corpora cavernosa and is connected to a pump. When the pump is squeezed, and released several times (recycling), the cylinders are filled with sterile normal saline, simulating the corpora cavernosa blood filing during physiologic erection. There are three types of inflatable devices: single-, two- and three-piece prostheses, based on whether the devices have a small reservoir in the end of each cylinder (single-piece) or attached to the pump (two-piece); or a larger (separate) reservoir that is connected to the pump (three-piece). Hence, the rigidity and girth achieved by the three-piece devices usually resemble a more natural penile erection.
Single-piece IPP
During the mid-1980s, two different one-piece IPP were introduced: Surgitek’s Flexi-Flate implant (Surgitek, Racine, WI, USA) (26) and AMS Hydroflex prosthesis (American Medical Systems, Minnetonka, MN, USA) (27). The Surgitek Flexi-Flate prosthesis (28) is composed of two hydraulic cylinders and the body of the device contains two chambers, an outer reservoir chamber and the inner pore chamber. The AMS Hydroflex prosthesis, consists of two cylinders in two fixed girth widths and various lengths with attachable rear tip extenders (29). It has an incorporated reservoir in proximal end and the inflate-deflate pumps in the distal end, that allows for transfer a small volume of fluid into a non-distensible central core.
The AMS Hydroflex was soon succeeded by the AMS Dynaflex in the 1990s, which is basically the same device but with multiple channels connecting the pump and reservoir portions, thus providing better rigidity comparable to a malleable device (30). When these devices are deflated, the central core will collapse and produce some degree of penile flaccidity. Nonetheless, these devices have been shown to be inferior to two- or three-piece inflatable prostheses with regards to mechanical reliability and patient satisfaction rate (31).
Two-piece IPP
In late 1980s, Frein (together with Mentor Corporation) developed and marketed a two-piece hydraulic implant called “GFS prosthesis” (stands for girth, flaccidity and simplicity) (32). This Mentor GFS prosthesis is a two-piece IPP consisting of paired cylinders connected by tubing to both reservoir and pump which are combined into a “resipump” placed in the scrotum. The cylinders can expand to a fixed girth and are provided in two width sizes, while the reservoir and pump are combined into a “resipump” placed in the scrotum. However, high complication rates such as poor mechanical reliability and infection rate, have led to a second-generation model, the Mark II, with less connection components (33). Around the same time, Surgitek, Inc., introduced another two-piece IPP called Uniflate 1000 where the device is filled through a self-sealing penetrable port on the bottom of the resipump, but the cylinders have two layers, an outer silicone layer and an inner Dacron layer, creating two chambers in each cylinders, with the outer chambers adding further girth to the cylinder. This device did not receive FDA approval and tubing fracture remains a constant source of mechanical failure.
In 1990s, AMS introduced Ambicor, a two-piece prefilled and pre-connected IPP consisting of a pair of cylinders and a pump composed of silicon elastomers. During prosthesis recycling, the pump transfers the solution from small reservoirs located at the proximal end of each cylinder, into each cylinder shaft, thereby causing an erection. The Ambicor two-piece penile prosthesis remains in market today (34).
Three-piece IPP
The original three-piece IPP prototype was developed by Scott and co-workers, and the original device consisted of two pumps (placed in each hemiscrotum), two cylinders, and a fluid reservoir (1). Recycling of the IPP involves squeezing one pump to inflate the device while squeezing the other pump results in flaccidity of the penis. The pumping mechanism was subsequently changed to a single pump containing an inflate and a deflate mechanisms. The original surgical description of preparation and insertion of these cylinders was cumbersome and involves the use of isotonic contrast solution and extreme cold freeze technology. The introduction of Furlow introducer (by Furlow) and Dilamez inserter (by Scott) in 1980 facilitated cylinder placement thus avoiding the complex cylinder freezing process, while subsequent development of angled tubing connectors (by Furlow) circumvents the need to tunnel the tubing in and out of the inguinal canal (35,36).
At present Boston Scientific AMS 700 series and Coloplast Titan IPP owns the majority share of the commercial inflatable prosthesis market (Table 2). Other companies who have developed and attempted to market three-piece IPP include Bard and Zephyr. The CR Bard Company (Murray Hill, New Jersey, USA) in 1985 developed a Bard IPP which consisted of two single-layer silicone cylinders, a disk-shaped titanium reservoir with a sealed Freon compartment, and an open saline fluid compartment and an activation button that controlled saline flow between the reservoir and the cylinder. Unfortunately, this product failed to gain purchase among surgeons in North America. The Zephyr ZSI 475 (Zephyr Surgical Implants SRAL, Geneva, Switzerland) was marketed in 2012 and has managed to achieve some regional success in Europe. More recently the company introduces a world first three-piece IPP for transgender patient with a larger more glans-like tip and a plate made from stainless steel and silicone on the proximal part which can be fixed securely to the pubic bone.

Full table
While the first IPP prototype was introduced in 1972, various innovations and developments in cylinder design and technology were made by the AMS company between 1973 and 1990 (37). The Fluid Transfer System 2 (FTS2) device consisted of two cylinders of silicone sheets reinforced with Dacron polyester fabric, two pumps for separate inflation and deflation mechanisms, and a pancake reservoir. Around 15 devices were implanted between 1973 and 1974 before the product was discontinued. The penile prosthesis-diameter (PPD) cylinders was introduced in 1975 with larger tubing size, molded dome front tip and more conical rear tip. The penile prosthesis-rod (PPR) cylinders designed in 1976 had a flexible silicone reinforcing rod placed inside the cylinder. The penile prosthesis-suture (PPS) cylinders was marketed between 1977 and 1983, and consisted of a distal “nipple” tip for device placement using Furlow inserter tool, suture, and needle. The penile prosthesis-huge (PPH) cylinder manufactured between 1997 and 1983, had an increase outer diameter for larger corpora, while the penile prosthesis-thick (PPT) and penile prosthesis-non-distensible (PND) devices introduced between 1983 and 1987 consisted of thicker silicone wall and polyester fabric between the two cylinders as well as PTFE sleeve to reduce cylinder aneurysm. The initial “PPT” prosthesis received several modifications to the proximal cylinder with longer proximal non-inflatable segment and a thicker wall to help minimise aneurysm formation by allowing for less friction damage between the tubing and cylinder wall. Changes to the reservoir system were made between 1972 and 1997, from the initial “pancake” reservoir to a spherical reservoir with silicone shell and an adaptor with standpipe tubing. The prosthesis pump also underwent several versions from IPI 741 to IPI 742(a), with incorporation of combination inflate and deflate pump mechanisms, first poppet and spring design, and inverted pump bulb. Between 1980s to 1990s, various modifications to the IPP were made to enhance the product such as rear tip extenders, kink resistant tubing, heat set cylinder fabric, Window Quick Connect connectors and connector assembly tool.
The current three-piece IPP manufactured by the Boston Scientific (previously AMS company) has three variations on the AMS 700 series: the AMS 700 LGX (previously Ultrex), AMS 700 CX and AMS 700 CXR. The girth-only expanding cylinder was termed “controlled expansion” (“CX”) and the length and girth expanding cylinder was called the “Ultrex” (38,39) and later became the “length girth expansion” (“LGX”) in 2006 (40,41). A narrower version of the CX, termed the “controlled expansion modified” (“CXM”) and subsequently the “controlled expansion restricted” (“CXR”), was later added for the narrower penis of the oriental market, but was found to be useful in patients with corporal fibrosis (42). A three-layered fabric was introduced in AMS cylinders, an inner silicone layer, a middle woven Dacron and Lycra layer, and an outer silicone layer to minimise cylinder aneurysm formation and mechanical failure (43). A unidirectional weave of the Dacron and Lycra results in a girth and length expansion of the cylinder when filled with fluid. The outer silicone layer prevents ingrowth of tissues into the cloth material of the middle layer. The CX and CXR have a unidirectional weave to their fabric allowing for girth expansion only, whereas the LGX has a bidirectional weave permitting expansion in length and girth.
In early 2000s, four further product innovations were added to the AMS 700 series. An additional parylene coating was added to the surface of the silicone and this micropolymer increases the lubricity of the silicone and wear resistant, further diminishes the risk of cylinder aneurysmal dilation. A lock-out valve was incorporated into the pump to prevent auto-inflation of the penile cylinders against extensive force or sudden elevated pressure within the reservoir. AMS introduced the first permanent antibiotic eluting implant, the InhibiZone, which consists of a formulation of minocycline hydrochloride and rifampicin, that is impregnated onto the outer surface of the device resulting in a marbled yellow-orange trace or modelled effect (44). Published literature showed that combination of minocycline and rifampicin can act synergistically to prevent bacteria colonization and is particularly effective against staphylococcus, the most common cause of IPP infection. The introduction of InhibiZone antimicrobial coating significantly reduces prosthesis-related infection rate when comparing InhibiZone-coated and uncoated prostheses (45). Changes were also made to the pump between 2004 and 2006 with the initial tactile pump which was easier to grasp and allowed for transfer a larger volume of fluid per squeeze (46), and later the momentary squeeze (MS) pump which eases pump deflation by not requiring the patient to hold the deflation button throughout deflation, but only requiring a quick squeeze of the button. The MS pump is also smaller than prior AMS pumps, making it easier to grasp and conceal in the scrotum (47). In 2010, AMS introduced the conceal reservoir which has a flat “pan-cake” like configuration as compared to traditional round sphere reservoir when filled with saline, providing better concealment especially if the reservoir is placed ectopically.
In 1982 Mentor Corporation introduced a competing three-piece inflatable implant, Mentor Alpha-1 IPP to the AMS penile prostheses (48). The cylinders and reservoir were composed of polyurethane and the pump and tubing were composed of silicone. A decade later, Mentor presented Bioflex, which was reported to be a polyether urea urethane elastomer, a very resilient and robust material (49). Mentor cylinders in testing appear to be more abrasion resistant than silicone cylinders.
Over the past two decades, the Coloplast Titan IPP has also benefitted from several innovative modifications that has improved the device function, reliability and durability (50). In 2000, a reservoir lockout valve was added to the reservoir to prevent prosthesis auto-inflation, reducing auto-inflation rate from 11% in patients with original reservoir to 1.3% in those with a lockout valve-equipped reservoir (51). In 2002, hydrophilic coating was introduced which decreases bacterial attachment and binds antibiotics, facilitating the use of surgeon’s antibiotic of choice and reducing infection by 50% (52). A unique advantage of the Titan’s hydrophilic coating is the ability to choose any water-based substance to adsorb to the device, potentially conferring an antimicrobial advantage in the use of any substance. Other modifications over the years such as Titan Narrow Base and Titan XL cylinders, twist-on rear tip extenders for prosthesis size adjustment, changing the tubing connector from crimp variety to slip-on variety, zero-degree junction between the cylinders and tubing to facilitate intracorporal cylinder placement, and soft moulded cylinder tips that more closely approximate human anatomy, have further enhanced the Titan IPP and improve its mechanical reliability and durability. The development of new pump models such as the one-touch release (OTR) pump and later Titan Touch pump improve the overall recycling and handling of penile pump (53,54). More recently the Coloplast Cloverleaf reservoir was introduced with the lock-out valve mechanism resided at the reservoir to minimise auto-inflation, allowing for better prevention of sudden change in pressure and has been approved for ectopic reservoir placement in anatomically compromised patients (55,56).
Conclusions
Penile prosthesis surgery remains an effective, safe and durable treatment option for male ED. Strict patient selection and counselling, strict adherence to antimicrobial prophylaxis, and surgical practice are paramount to ensure low complication and high patient satisfaction rates. Since the introduction of IPP by Scott in 1973, the surgical landscape for penile prosthesis implant has changed dramatically. Malleable penile prosthesis is usually cheaper than IPP, and the insertion of malleable device is often easier because there is no need to place a reservoir or a pump. In fact, malleable penile prosthesis often provides an ideal option in men who are physically handicapped with poor hand dexterity or limited fingers movement, complain of muscle fatigue (as in neurological disorders), or have limited reach or range of mobility (e.g., spinal patients). However, the IPP is considered a superior option to malleable prosthesis as it produces penile rigidity and flaccidity that closely replicates a normal penile erectile function. Improvement in IPP materials provides critical enhancement to the device resulting in revision free survival from mechanical failure at greater than 90% and an overall IPP survival in the high 80% at 3 to 5 years (42,43,57,58). The use of antibiotic coatings such as InhibiZone and hydrophilic material, have minimized the risk of infection to 1–2% (44,45,52). Mulcahy’s innovative approach at salvage and rescue reimplantation has proven to be a highly successful approach to early infection or subclinical infected penile prosthesis implant (59,60). New pancake-like or flat reservoirs, designed to decrease palpability, allow for ectopic/submuscular and/or subfascia placement (55,56), where traditional retropubic placement may be difficult (e.g., neobladder) and can be associated with vascular, bladder and bowel complications (e.g., renal transplant, pelvic radiation or previous abdominopelvic surgery).
In the current era of consumerism, patients are demanding higher quality products, and the continued investment by prosthesis companies in research and design development, have resulted in more innovative, superior and newer generation prosthesis. Throughout the years, technological advances in penile prostheses such as kink resistant tubing, rear tip extenders, lock-out valve, tactile pump, polypropylene cylinder coating and antibiotic impregnated device have improved the mechanical reliability and durability of these penile prostheses (61). Despite these scientific enhancements and excellent long term functional and patient outcomes, a small percentage of patients remain dissatisfied with the procedure and penile prosthesis. Although most of these dissatisfactions with the surgical procedure may be related to technical aspects such as intraoperative complications or poor surgical outcomes, factors such as poor preoperative informed consent and suboptimal patient selection can contribute to high risk for patient dissatisfaction. Other reasons for patient dissatisfaction following penile prosthesis surgery include loss of perceived length, lack of glanular engorgement, unnaturalness as perceived by the partner, or an overall sexual dissatisfaction by the patient and/or partner (62).
While the ideal penile prosthesis is probably yet to be developed, scientific advances in prosthesis design, device technology and surgical techniques have made the penile prosthesis more natural, durable and reliable device. Despite the introduction of pro-erectile pharmacological agents and the greater understanding of the pathophysiology of ED, penile prosthesis implant continues to play an important role in the management of ED. The continued partnership and synergistic relationship between surgeons and device manufacturers, to learn of surgeons’ and patients’ needs, and to preview with them proposed new devices and revisions, is paramount to scientific progress that has been made and future technological innovations in penile prosthesis implant.
Acknowledgements
The author thanks Boston Scientific AMS Men’s Health and Coloplast companies for sharing historical data.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Scott FB, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology 1973;2:80-2. [Crossref] [PubMed]
- Brindley GS. Cavernosal alpha-blockade: a new technique for investigating and treating erectile impotence. Br J Psychiatry 1983;143:332-7. [Crossref] [PubMed]
- Virag R. Intracavernous injection of papaverine for erectile failure. Lancet 1982;2:938. [Crossref] [PubMed]
- Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998;338:1397-404. [Crossref] [PubMed]
- Gee WF. A history of surgical treatment of impotence. Urology 1975;05:401-5. [Crossref] [PubMed]
- Frumkin AP. Reconstruction of the male genitalia. Am Rev Soviet Med 1944;2:14-7.
- Bergman RT, Howard AH, Barnes RW. Plastic reconstruction of the penis. J Urol 1948;59:1174-86. [PubMed]
- Goodwin WE, Scott WW. Phalloplasty. J Urol 1952;68:903-8. [PubMed]
- Beheri GE. Surgical treatment of impotence. Plast Reconstr Surg 1966;38:92-7. [Crossref] [PubMed]
- Lash H. Silicone implant for impotence. J Urol 1968;100:709-10. [PubMed]
- Lash H, Zimmerman DC, Loeffler RA. Silicone implantation: inlay method. Plast Reconstr Surg 1964;34:75-80. [Crossref] [PubMed]
- Pearman RO. Treatment of organic impotence by implantation of a penile prosthesis. J Urol 1967;97:716-9. [PubMed]
- Pearman RO. Insertion of a silastic penile prosthesis for the treatment of organic sexual impotence. J Urol 1972;107:802-6. [PubMed]
- Small MP, Carrion HM, Gordon JA. Small-Carrion penile prosthesis. New implant for management of impotence. Urology 1975;5:479-86. [Crossref] [PubMed]
- Martinez DR, Terlecki R, Brant WO. The Evolution and Utility of the Small-Carrion Prosthesis, Its Impact, and Progression to the Modern-Day Malleable Penile Prosthesis. J Sex Med 2015;12 Suppl 7:423-30. [Crossref] [PubMed]
- Falcone M, Rolle L, Ceruti C, et al. Prospective analysis of the surgical outcomes and patients' satisfaction rate after the AMS Spectra penile prosthesis implantation. Urology 2013;82:373-6. [Crossref] [PubMed]
- Hsu GL, Chen HS, Huang SJ. Does tunica anatomy matter in penile implant? Transl Androl Urol 2015;4:406-12. [PubMed]
- Casabé AR, Sarotto N, Gutierrez C, et al. Satisfaction assessment with malleable prosthetic implant of Spectra (AMS) and Genesis (Coloplast) models. Int J Impot Res 2016;28:228-33. [Crossref] [PubMed]
- Finney RP. New hinged silicone penile implant. J Urol 1977;118:585-7. [PubMed]
- Jonas U, Jacobi GH. Silicone-silver penile prosthesis: description, operative approach and results. J Urol 1980;123:865-7. [PubMed]
- Jonas U. Silicone-silver penis prosthesis (Jonas-Eska), long-term experiences. A critical assessment. Urologe A 1991;30:277-81. [PubMed]
- Fathy A, Shamloul R, AbdelRahim A, et al. Experience with Tube (Promedon) malleable penile implant. Urol Int 2007;79:244-7. [Crossref] [PubMed]
- Patwardhan SK, Shah R, Kulkarni V, et al. Shah's Indian penile prosthesis placement after phallic reconstruction with radial forearm flap. Indian J Urol 2008;24:107-8. [Crossref] [PubMed]
- Huisman TK, Macintyre RC. Mechanical failure of OmniPhase penile prosthesis. Urology 1988;31:515-6. [Crossref] [PubMed]
- Mulcahy JJ. The OmniPhase and DuraPhase penile prostheses. Urol Clin North Am 1989;16:25-31. [PubMed]
- Randrup ER. Clinical experience with 180 inflatable penile prostheses. South Med J 1995;88:47-51. [Crossref] [PubMed]
- Riehmann M, Gasser TC, Bruskewitz RC. The Hydroflex penile prosthesis: a test case for the introduction of new urological technology. J Urol 1993;149:1304-7. [PubMed]
- Stanisic TH, Dean JC, Donovan JM, et al. Clinical experience with a self-contained inflatable penile implant: the Flexi-Flate. J Urol 1988;139:947-50. [PubMed]
- Kabalin JN, Kessler R. Experience with the Hydroflex penile prosthesis. J Urol 1989;141:58-9. [PubMed]
- Anafarta K, Yaman O, Aydos K. Clinical experience with Dynaflex penile prostheses in 120 patients. Urology 1998;52:1098-100. [Crossref] [PubMed]
- Wilson SK, Cleves M, Delk JR 2nd. Long-term results with Hydroflex and Dynaflex penile prostheses: device survival comparison to multicomponent inflatables. J Urol 1996;155:1621-3. [Crossref] [PubMed]
- Fein RL. The G.F.S. Mark II inflatable penile prosthesis. J Urol 1992;147:66-8. [PubMed]
- Fein RL. GFS Mark II inflatable penile prosthesis: four-year clinical study. Urology 1994;43:209-13. [Crossref] [PubMed]
- Levine LA, Estrada CR, Morgentaler A. Mechanical reliability and safety of, and patient satisfaction with the Ambicor inflatable penile prosthesis: results of a 2 center study. J Urol 2001;166:932-7. [Crossref] [PubMed]
- Henry GD. Historical review of penile prosthesis design and surgical techniques: part 1 of a three-part review series on penile prosthetic surgery. J Sex Med 2009;6:675-81. [Crossref] [PubMed]
- Wilson SK, Delk JR 2nd. Historical advances in penile prostheses. Int J Impot Res 2000;12 Suppl 4:S101-7. [Crossref] [PubMed]
- Boston Scientific. Penile prosthesis implant. Available online: http://www.bostonscientific.com/en-US/medical-specialties/urology.html
- Liberman SN, Gomella LG, Hirsch IH. Experience with the Ultrex and Ultrex Plus inflatable penile prosthesis: new implantation techniques and surgical outcome. Int J Impot Res 1998;10:175-9. [Crossref] [PubMed]
- Montague DK, Lakin MM. Early experience with the controlled girth and length expanding cylinder of the American Medical Systems Ultrex penile prosthesis. J Urol 1992;148:1444-6. [PubMed]
- Mulcahy JJ. Use of CX cylinders in association with AMS700 inflatable penile prosthesis. J Urol 1988;140:1420-1. [PubMed]
- Negro CL, Paradiso M, Rocca A, et al. Implantation of AMS 700 LGX penile prosthesis preserves penile length without the need for penile lengthening procedures. Asian J Androl 2016;18:114-7. [Crossref] [PubMed]
- Daitch JA, Angermeier KW, Lakin MM, et al. Long-term mechanical reliability of AMS 700 series inflatable penile prostheses: comparison of CX/CXM and Ultrex cylinders. J Urol 1997;158:1400-2. [Crossref] [PubMed]
- Salem EA, Wilson SK, Neeb A, et al. Mechanical reliability of AMS 700 CX improved by parylene coating. J Sex Med 2009;6:2615-20. [Crossref] [PubMed]
- McKim SE, Carson CC 3rd. AMS 700 inflatable penile prosthesis with InhibiZone. Expert Rev Med Devices 2010;7:311-7. [Crossref] [PubMed]
- Carson CC 3rd. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol 2004;171:1611-4. [Crossref] [PubMed]
- Delk J, Knoll LD, McMurray J, et al. Early experience with the American Medical Systems new tactile pump: results of a multicenter study. J Sex Med 2005;2:266-71. [Crossref] [PubMed]
- Knoll LD, Henry G, Culkin D, et al. Physician and patient satisfaction with the new AMS 700 momentary squeeze inflatable penile prosthesis. J Sex Med 2009;6:1773-8. [Crossref] [PubMed]
- Merrill DC. Mentor inflatable penile prostheses. Urol Clin North Am 1989;16:51-66. [PubMed]
- Merrill DC, Javaheri P. Mentor inflatable penile prosthesis. Preliminary clinical results in 30 patients. Urology 1984;23:72-4. [Crossref] [PubMed]
- Coloplast. Penile prosthesis implant. Available online: https://www.coloplast.us/
- Wilson SK, Henry GD, Delk JR Jr, et al. The mentor Alpha 1 penile prosthesis with reservoir lock-out valve: effective prevention of auto-inflation with improved capability for ectopic reservoir placement. J Urol 2002;168:1475-8. [Crossref] [PubMed]
- Wolter CE, Hellstrom WJ. The hydrophilic-coated inflatable penile prosthesis: 1-year experience. J Sex Med 2004;1:221-4. [Crossref] [PubMed]
- Shaw T, Garber BB. Coloplast titan inflatable penile prosthesis with one-touch release pump: review of 100 cases and comparison with genesis pump. J Sex Med 2011;8:310-4. [Crossref] [PubMed]
- Ohl DA, Brock G, Ralph D, et al. Prospective evaluation of patient satisfaction, and surgeon and patient trainer assessment of the Coloplast titan one touch release three-piece inflatable penile prosthesis. J Sex Med 2012;9:2467-74. [Crossref] [PubMed]
- Ziegelmann MJ, Viers BR, Lomas DJ, et al. Ectopic Penile Prosthesis Reservoir Placement: An Anatomic Cadaver Model of the High Submuscular Technique. J Sex Med 2016;13:1425-31. [Crossref] [PubMed]
- Chung PH, Morey AF, Tausch TJ, et al. High submuscular placement of urologic prosthetic balloons and reservoirs: 2-year experience and patient-reported outcomes. Urology 2014;84:1535-40. [Crossref] [PubMed]
- Wilson SK, Delk JR, Salem EA, et al. Long-term survival of inflatable penile prostheses: single surgical group experience with 2,384 first-time implants spanning two decades. J Sex Med 2007;4:1074-9. [Crossref] [PubMed]
- Chung E, Van CT, Wilson I, et al. Penile prosthesis implantation for the treatment for male erectile dysfunction: clinical outcomes and lessons learnt after 955 procedures. World J Urol 2013;31:591-5. [Crossref] [PubMed]
- Mulcahy JJ. Long-term experience with salvage of infected penile implants. J Urol 2000;163:481-2. [Crossref] [PubMed]
- Mulcahy JJ. Current approach to the treatment of penile implant infections. Ther Adv Urol 2010;2:69-75. [Crossref] [PubMed]
- Pastuszak AW, Lentz AC, Farooq A, et al. Technological Improvements in Three-Piece Inflatable Penile Prosthesis Design over the Past 40 Years. J Sex Med 2015;12 Suppl 7:415-21. [Crossref] [PubMed]
- Trost LW, Baum N, Hellstrom WJ. Managing the difficult penile prosthesis patient. J Sex Med 2013;10:893-906. [Crossref] [PubMed]


