Intralesional collagenase clostridium histolyticum study provides real-world analysis
Yang and Bennett’s report (1) offers further support for the use of intralesional collagenase clostridium histolyticum (CCH) to improve both subjective symptoms and objective measures in men with Peyronie’s disease (PD). The study included men who would not have been eligible for the IMPRESS (2) trials, including those with active disease, hourglass configurations, ventral deformities, and no upper limit in initial curvature measurements. CCH therapy is currently contraindicated for patients with ventral plaques and hourglass deformities (3). Even with the inclusion of these men, average penile curvature in Yang and Bennett’s report decreased 32.4% compared to the IMPRESS trials, in which the average curvature decreased 34%, with similar efficacy. The percentage of men who had discernable changes to CCH therapy was 60.8% in the IMPRESS trials, while Yang and Bennett observed that 79.6% of men treated with CCH therapy had responded with a decrease in curvature. These results suggest that intralesional CCH therapy may be applied more broadly to PD patients than it is currently approved for.
Additionally, the median number of injections completed in Yang and Bennett’s study was six, indicating that the median number of cycles completed was three. In a subset analysis of those men who completed all four cycles, the average curvature decrease was 38.7%. Given that the majority of the patients had not completed the standard four cycles of the treatment, the question remains if further improvement would have been noted upon completion of all cycles.
Intralesional injection of CCH is associated with hematoma formation and, most seriously, corporal rupture. It is estimated that one in three high-volume CCH providers will witness a CCH-associated corporal rupture (4). While efficacy remained comparable to that seen in the IMPRESS trials, the percentage of patients experiencing serious adverse events—defined as penile bruising, penile hematoma, or penile fracture—was somewhat higher in this study at 10.2% (5/49). In the IMPRESS trials 1.1% (6/551) of patients were labeled as having serious adverse events. However, the definition of adverse events differed from those of the IMPRESS trials, as well as patient inclusion criteria and penile modeling methodology post-injection. In the study by Yang and Bennett, only one out of five underwent surgical correction, whereas the IMPRESS trials identified four out of six patients who had surgical intervention. This difference is likely, in part, due to the evolving change in practice of managing corporal rupture conservatively, rather than surgically. However, it will also be important to determine whether the broad inclusion criteria, methodology, or a simple sampling error had any effect on complication rate.
This study provides a contemporary real-world analysis of men treated with intralesional CCH by providers with significant experience with PD. This study by Yang and Bennett is a notable contribution to our overall understanding of CCH therapy, but also serves to re-define critical questions that remain somewhat ambiguous. Table 1 offers a summary of topics that mandate further investigation in order to achieve the goals of maximum patient efficacy and safety of the use of intralesional CCH therapy in men suffering with PD.
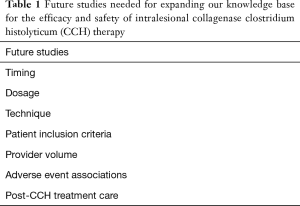
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: Hellstrom WJ served as the primary investigator for the IMPRESS trials and is currently on a speaker's bureau and advisory board for Endo Pharmaceuticals. Haney NM and DeLay KJ have no conflicts of interest to declare.
References
- Yang KK, Bennett N. Peyronie's Disease and Injectable Collagenase Clostridium histolyticum: Safety, Efficacy, and Improvements in Subjective Symptoms. Urology 2016;94:143-7. [Crossref] [PubMed]
- Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 2013;190:199-207. [Crossref] [PubMed]
- Yafi FA, Hatzichristodoulou G, DeLay KJ, et al. Review of Management Options for Patients With Atypical Peyronie's Disease. Sex Med Rev 2016. [Epub ahead of print].
- Yafi FA, Anaissie J, Zurawin J, et al. Results of SMSNA Survey Regarding Complications Following Intralesional Injection Therapy With Collagenase Clostridium Histolyticum for Peyronie's Disease. J Sex Med 2016;13:684-9. [Crossref] [PubMed]


