Testosterone replacement therapy: role of pituitary and thyroid in diagnosis and treatment
Symptoms of hypogonadism in men
Consultation and prescription for alleged testosterone deficiency in adult men has increased substantially in recent years. Symptoms of androgen deficiency are numerous and shown in Table 1. The prevalence of androgen deficiency increases with age, from about 0.1% of men aged 40–49 to about 3–5% aged 60–79 without other risk factors; this number increases in the presence of comorbidities such as type 2 diabetes mellitus or obesity (1). A large epidemiological study, the Massachusetts Male Aging Study (MMAS), reported a prevalence of sexual dysfunction of up to 34.8% in men 40–70 years old (2). In rodent models, Ferrini et al. describe coexistent apoptosis in the hypothalamus and gonads over time, a speculative reason for age-related androgen decline in these animals (3). Other hormones are known to undergo age-related decline as well, including thyroxine and growth hormone, the adaptive reasons for which are not well understood (4).

Full table
Timing of onset of androgen deficiency will dictate the pathophysiologic effects and exam findings, and other diagnoses such as vascular disease should be considered given overlaps in clinical presentation. Factors that may contribute to the development of testosterone deficiency include chronic illness, obesity, type 2 diabetes mellitus, depression, treatment of genitourinary and other cancers, and medications which interfere with testosterone production and/or metabolism (e.g., opiates, glucocorticoids). Promptly recognizing, accurately diagnosing, and sufficiently treating hypogonadism in men may lead to increased personal well-being as well as more optimal relationships with sexual partners, both of which contribute significantly to overall quality of life. Whether inappropriate treatment when the diagnosis of hypogonadism is insufficiently proven is potentially injurious is a matter of controversy.
Proper laboratory diagnosis
Physiologic testosterone production is known to follow a diurnal rhythm, with higher serum concentrations early in the morning, and lower concentrations later in the day. Therefore, most guidelines recommend measuring total testosterone early morning as the initial test to avoid false diagnoses. It is important to remember that sleep disturbance, due to night shift work or for any reason, will disrupt this diurnal pattern. The strict timing of measurements in men over 60 years old is less important due to blunting of circadian rhythm with age (5). The diagnosis of testosterone deficiency should be made only in a patient with specific symptoms and at least two unequivocally low testosterone levels, as up to 33% of men over 45 years old can have low testosterone on a single measurement (6,7). Variation among individuals in the threshold at which symptoms occur may also play a role. Testosterone should not be evaluated during acute illness due to disruption of homeostasis.
Due to a higher frequency of hypogonadism, measurement of serum testosterone may be considered regardless of the presence of symptoms in the following groups: patients with HIV-associated weight loss, history of sellar radiation or known sellar mass, end stage renal disease on hemodialysis, moderate to severe chronic obstructive pulmonary disease (COPD), type 2 diabetes mellitus, and history of osteoporosis/fragility fracture (8) (Table 2).

Full table
When total testosterone levels are “low-normal” in patients with obesity, chronic illness, liver disease or thyroid disease further evaluation with sex hormone binding globulin (SHBG) levels and free testosterone by reliable assay should be performed in morning samples (9,10). Normally, 0.5–3% of testosterone is free and therefore readily bioavailable, whereas 30% is tightly bound to SHBG and the rest more loosely bound to other serum proteins with varying degrees of bioavailability (11). Several other endocrine and non-endocrine diseases can affect SHBG levels. Situations associated with increased SHBG are aging, hyperthyroidism, using anticonvulsants and estrogens; on the other hand, hypothyroidism, obesity, hyperprolactinemia, and insulin resistant states including type 2 diabetes mellitus, growth hormone excess, use of glucocorticoids or androgens/anabolic steroids and endogenous hypercortisolemia can decrease SHBG and lead to low serum total testosterone (12,13).
Once a low testosterone level has been unequivocally established in the presence of symptoms of hypogonadism, further endocrinological workup will help ascertain the etiology of androgen deficiency. This, along with the patient’s potential goals for fertility, will help guide therapeutic decisions.
Distinguishing between primary and secondary hypogonadism
In men >95% of testosterone is produced in the Leydig cells of the testes in response to luteinizing hormone (LH) from the anterior pituitary gland, a process which is dependent on gonadotrophin-releasing hormone (GnRH) stimulation from the hypothalamus. Some testosterone is derived from conversion of adrenal androgens, the amount of which is not sufficient to counteract low testosterone levels from hypogonadism. GnRH is normally released in a pulsatile fashion, and disruption of this pulsatile pattern leads to desensitization of the gonadotrophic cells with resultant decreased release of LH and FSH. To determine whether hypogonadism is primary (testicular) or secondary (pituitary and/or hypothalamic, also termed hypogonadotrophic) in origin, serum FSH and LH levels should be measured. In primary hypogonadism FSH and LH will be elevated due to lack of the normal negative feedback that testosterone and its derivatives dihydrotestosterone (DHT) and estradiol have on the hypothalamic-pituitary axis (14). Most guidelines recommend karyotype analysis to evaluate for Klinefelter syndrome (47 XXY) in men with primary hypogonadism, whereas most other etiologies of primary hypogonadism can be ascertained from thorough clinical history and examination (8). Other causes of primary hypogonadism include prior therapy for testicular tumor, acquired anorchia, uncorrected cryptorchidism, HIV, orchitis, toxins, and other genetic syndromes including Noonan syndrome and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (8,15).
Pituitary issues
When FSH and LH are either low or inappropriately normal in the face of low serum testosterone this points to a problem at the hypothalamic or pituitary level. Potential causes are numerous, including tumorous, granulomatous or infiltrative disease involving the hypothalamus and/or pituitary, Kallman syndrome (associated sometimes with anosmia and renal abnormalities due to mutated kal-1 gene), congenital adrenal hyperplasia, untreated sleep apnea, various drugs (e.g., opiates, marijuana, heroin) and GnRH deficiency (16,17). When no other cause is identified, the diagnosis may be designated idiopathic hypogonadotrophic hypogonadism, which has a reported prevalence of 0.025% (18).
Hyperprolactinemia, which accounts for up to 5% of cases of secondary hypogonadism, can result from a prolactin-secreting pituitary adenoma (prolactinoma), pressure on the pituitary stalk by any pituitary lesion (interrupting the normal dopamine inhibition of prolactin secretion), medications which affect prolactin secretion (e.g., risperidone, metoclopramide), hypothyroidism, and chronic renal failure (19,20). Pituitary adenomas, whether or not they are functional, may cause local compression of gonadotrophs leading to hypogonadism. GnRH deficiency can result from insults to the hypothalamus due to medications, toxins, trauma or systemic disease (8). The recommended initial workup for secondary hypogonadism includes a prolactin level and transferrin saturation with serum ferritin per most guidelines, the latter to rule out iron overload (hemochromatosis). Investigation of other pituitary axes and the need for pituitary imaging is based on clinical suspicion and results of initial evaluation.
Differentiating hypothalamic versus pituitary etiology of idiopathic hypogonadotrophic hypogonadism may have implications for treatment in men who seek fertility. Snyder et al. [1979] evaluated 10 men with presumed pituitary or hypothalamic hypogonadism (all with testosterone <175 ng/dL) and infused GnRH over 4 hours daily for one week (21). Those men with pituitary etiologies (adenoma, hemochromatosis) of hypogonadism did not have robust increases in LH in response to prolonged GnRH pulses signifying inadequate gonadotroph function, whereas those with hypothalamic pathology (Kallman syndrome, sarcoidosis, or Hand-Schüller-Christian disease) had incremental increases in LH into the normal range (21). This phenomenon has been confirmed by others, and translated into therapeutic developments for both male and female infertility (22,23). Therefore, if a man with secondary hypogonadism originating in the hypothalamus desires to have children, treatment with pulsatile GnRH or human chorionic gonadotropin therapy should be considered (24-26). If fertility is not desired, and there are no contraindications as laid out in the guidelines, testosterone replacement therapy is considered the treatment of choice.
Importantly, several conditions can lead to a combined primary and secondary hypogonadism in men, including glucocorticoid use, alcohol abuse, hemochromatosis, sickle cell disease, granulomatous disease such as sarcoidosis, and thalassemia (17,27). Gonadotrophin levels and spermatogenesis will be variable, based on the predominant primary versus secondary hypogonadal effect of the insult.
Hyperprolactinemia
Hyperprolactinemia is known to affect the natural pulsatile secretion of GnRH as well as the pituitary response to GnRH, which leads to decreased testosterone production and secretion by the Leydig cells (9). Sexual dysfunction may result, even with serum testosterone levels in the low normal range, though elevated prolactin can also lead to increased levels of free testosterone via decreased SHBG levels (28). Studies suggest that hyperprolactinemia, even in the presence of low-normal testosterone, leads to erectile dysfunction and decreased libido, and treatment with a dopamine agonist may improve symptoms without necessarily causing an increase in testosterone levels (29-31). The syndrome of hyperprolactinemia in men is more polymorphic and tends to be recognized later in life than in women (31). Prolactin receptors have been shown to be present on Leydig and Sertoli cells in mammalian testes, where physiologic levels of prolactin increase the number of LH and FSH receptors, among other effects (32). Hyperprolactinemia has been associated with a decrease in sperm mobility and fertilizing capabilities, both of which may be reversible with dopamine agonist treatment (31,33-36). Several studies have also suggested elevation of prolactin may detrimentally affect testosterone metabolism by 5-alpha reductase to the more potent androgen DHT (37,38). The mass effect of a prolactin-producing pituitary adenoma may also directly affect gonadotrophic production, though the hypogonadal effect is likely minor in the presence of hyperprolactinemia (31). In hypogonadal men with hyperprolactinemia, initial treatment should be directed towards normalizing serum prolactin levels and assessing improvement in serum testosterone and in hypogonadal symptoms prior to considering testosterone replacement.
Adrenal considerations
The incidence of hypogonadism in men with secondary adrenal insufficiency, or primary for that matter, is not well described. Secondary adrenal insufficiency is associated with low values of adrenal glucocorticoids without mineralocorticoid deficiency. One postulation is that the decreased dehydroepiandrosterone (DHEAS) levels, through pituitary-dependent and -independent mechanisms, in this population may lead to sexual dysfunction (low libido, loss of axillary/pubic hair), though this is more often studied in women and the effects in men are not well understood (17). Literature on the effects of exogenous steroids on hypogonadism in men is relatively scant. Dev et al. reported on three men in whom treatment with megestrol acetate for cancer-related cachexia was associated with both adrenal and gonadal suppression (39). There are admittedly, confounding effects in such patients, who are exposed to varying levels of opiate treatment and are often systemically ill, both of which can independently lead to testosterone deficiency (40). The glucocorticoid properties of megestrol likely lead to significant decrease in testosterone levels, and this medication has also been shown to increase prolactin levels in healthy men (41,42). Some studies have shown that megestrol decreases LH levels in both healthy men and cancer patients (42,43). MacAdams et al. have suggested that exogenous glucocorticoids may directly suppress GnRH release from the hypothalamus in men with COPD (44). It should also be noted that sudden withdrawal (without taper) of megestrol or other medications with glucocorticoid properties may cause acute adrenal insufficiency, which can compound symptoms of fatigue and low libido in patients with hypogonadism.
As noted above, exogenous glucocorticoids have been shown to contribute to testosterone deficiency in men in a dose-dependent fashion (44). In the presence of elevated endogenous glucocorticoids, as in Cushing disease, suppression of the hypothalamic-pituitary-gonadal axis has similarly been observed, though theories vary as regards suppression being a direct testicular or a hypothalamic-pituitary axis affect. A study of 12 men with Cushing disease showed that treatment of the condition led to reversal of pre-treatment hypogonadotrophic hypogonadism (45). The investigators suggested that decreased gonadotrophic response to GnRH in the untreated hypercortisolemic state was the cause of the hypogonadism. In another study of Cushing syndrome patients the gonadotrophin response to GnRH was variable, suggesting the effects of hypercortisolism may occur at variable degrees at all sites along the hypothalamic-pituitary-gonadal axis in this population (46). There is a relative paucity of literature on male patients with Cushing’s and hypogonadism, and that which exists is not recent, though the relationship is frequently observed in clinical practice (47,48).
Investigating pituitary function
In practice, the diagnosis of one endocrinopathy often prompts concern for additional ones, as the endocrine system is complex, with significant multi-organ interplay. Therefore, in patients with suspected androgen deficiency the clinical history and exam should incorporate evaluation for other possible hormonal imbalances. Evaluation of the other anterior pituitary axes is strongly recommended in patients with secondary hypogonadism when the total testosterone is as low as <150 ng/dL, and is also indicated in men with other clinical findings suspicious for either hypopituitarism or pituitary hyperfunction in the presence of less severe testosterone deficiency (8,19). As discussed above, all patients being evaluated for androgen deficiency should have their prolactin level measured. Clinical suspicion will dictate the need to rule out adrenal axis dysfunction, growth hormone dysfunction, and central thyroid dysfunction.
Screening for adrenal insufficiency can begin with an early morning (no later than 9 AM) blood draw for cortisol and ACTH. If morning sampling is not convenient, or its value compromised because of night-work, an ACTH stimulation test, using 0.25 mg cosyntropin (tetracosactide) intramuscularly, should be performed (49). We also recommend this test in patients whose early morning cortisol is <15 mcg/dL.
If Cushing syndrome is suspected screening should involve one or more of the following, depending on the level of suspicion: overnight 1 mg dexamethasone suppression test, midnight salivary cortisol on two consecutive nights, and/or 24 hour urine free cortisol measurement on two separate occasions (50).
If the patient is suspected of having either growth hormone deficiency or acromegaly, insulin-like growth factor-1 (IGF-1) and growth hormone should be assayed and further dynamic testing may be recommended if these values fall outside normal range (51).
While evaluating for central hypothyroidism or (much more rarely) central hyperthyroidism, a serum free thyroxine (T4) or free thyroxine index (FTI) must be done concomitantly with thyroid stimulating hormone (TSH) measurement, as the TSH alone can be misleading (52,53). In fact, we would say that relying solely on TSH measurement in this situation is one of the commonest errors we come across.
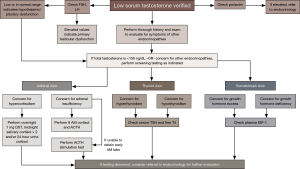
In the event that any of these screening tests are positive, these patients would benefit from referral to endocrinology (see Figure 1). These biochemical evaluations are necessary only when there is a reasonable suspicion of additional pituitary pathology in association with the presenting syndrome of hypogonadism.
The role of pituitary imaging
As with other recommendations in medicine, the decision to obtain pituitary imaging should be based on the likelihood of the result significantly contributing to clinical management. Pituitary “incidentalomas”, usually 6 mm diameter or less, are present on magnetic resonance imaging (MRI) in about 10% of the general population, and silent adenomas are found in up to 20% of autopsies (54). In our opinion pituitary MRI, preferably with and without contrast, should be performed in men with secondary hypogonadism who meet one of the following criteria (19,55-57): severe secondary hypogonadism (total testosterone level <150 ng/dL) with FSH and LH levels below normal, signifying a higher risk of pituitary pathology, persistent hyperprolactinemia, symptoms of headache or visual defects, or in the event that any other pituitary hormones are deficient on biochemical analysis.
Several recent studies have found that men with idiopathic hypogonadotrophic hypogonadism have pituitary pathology at a greater prevalence than the general population, some of which may be amenable to surgical treatment, even if they do not meet the criteria set forth above (58,59). However, given the expense of MRI and the fact that the vast majority of hypogonadotrophic hypogonadism cases initially assumed to be idiopathic turn out not to have any structural lesion, it is not uniformly recommended to obtain imaging unless one of the above situations is observed or if it is expected to affect management. If a pituitary lesion is observed in a man undergoing investigation for low testosterone, it is clear that evaluation of the other pituitary axes should be undertaken if not already done so. There is no role for pituitary imaging in straightforward cases of primary hypogonadism.
Thyroid issues
Both hypo- and hyperthyroidism can impinge on gonadal axis function in men, though a direct effect of thyroid hormones on the testis has not been demonstrated (60). The neuropsychiatric effects of thyroid dysfunction may independently contribute to sexual dysfunction in men with untreated thyroid disease; however, several biochemical relationships have also been observed. There is an increased gonadotroph response to GnRH in men with untreated hyperthyroidism compared to when treatment had rendered the men euthyroid, suggesting a possible role of thyroid hormone in sensitization of gonadotrophs (61). Hypothyroidism has been associated with low total testosterone levels, assumed to be due to low SHBG levels (13,62). However, low free testosterone levels, that normalize when the hypothyroidism is treated, have also been demonstrated (62). Hyperthyroidism increases concentrations of SHBG and total testosterone, but free testosterone levels are generally normal; it can also increase aromatization of testosterone to estrogen, which may also affect sexual function and lead to gynecomastia (9,13).
FSH and LH levels in men with thyroid dysfunction tend to be in the normal range, though in more severe hypothyroidism patients may exhibit elevated gonadotropins (62,63). As mentioned above, hypothyroidism can lead to hyperprolactinemia in a small portion of affected individuals, and in such cases treatment of the hypothyroidism can correct the prolactin levels and improve the symptoms of testosterone deficiency (62).
A study of 120 men with erectile dysfunction uncovered undiagnosed hypothyroidism in 5%, and hyperthyroidism in 1% (64). Hypothyroidism, whether primary or central, has been associated with erectile dysfunction and hypoactive sexual desire with unclear prevalence; levothyroxine replacement can improve symptoms and testosterone levels (62,65,66). Just as erectile dysfunction has been described in men with hypothyroidism; hyperthyroidism has also been associated with erectile dysfunction (67). A case series over 755 men with erectile dysfunction showed that >50% of those with hyperthyroidism (TSH <0.2 mU/L) had ejaculatory dysfunction (68). A prospective study of 23 thyrotoxic men showed decreased libido and abnormalities of sperm motility, both of which improved significantly after treatment of the hyperthyroidism, regardless of modality of treatment (69). Therefore, it is important to recognize and treat thyroid disease in patients presenting with testicular or sexual dysfunction prior to considering androgen therapy.
Conclusions
It is appropriate to entertain the possibility of additional endocrine dysfunction in any man presenting with hypogonadism. Addressing abnormalities in other pituitary axes may negate the need for testosterone replacement therapy in some cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wu FC, Tajar A, Beynon JM, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123-35. [Crossref] [PubMed]
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [PubMed]
- Ferrini M, Wang C, Swerdloff RS, et al. Aging-related increased expression of inducible nitric oxide synthase and cytotoxicity markers in rat hypothalamic regions associated with male reproductive function. Neuroendocrinology 2001;74:1-11. [Crossref] [PubMed]
- Vermeulen A. Andropause. Maturitas 2000;34:5-15. [Crossref] [PubMed]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab 1983;56:1278-81. [Crossref] [PubMed]
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762-9. [Crossref] [PubMed]
- Brambilla DJ, O'Donnell AB, Matsumoto AM, et al. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007;67:853-62. [Crossref] [PubMed]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536-59. [Crossref] [PubMed]
- Morales A, Buvat J, Gooren LJ, et al. Endocrine aspects of sexual dysfunction in men. J Sex Med 2004;1:69-81. [Crossref] [PubMed]
- Rosner W, Auchus RJ, Azziz R, et al. Position statement: utility, limita¬tions, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 2007;92:405-13. [Crossref] [PubMed]
- McClure RD. Endocrine investigation and therapy. Urol Clin North Am 1987;14:471-88. [PubMed]
- Buvat J, Maggi M, Guay A, et al. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med 2013;10:245-84. [Crossref] [PubMed]
- Dumoulin SC, Perret BP, Bennet AP, et al. Opposite effects of thyroid hormones on binding proteins for steroid hormones (sex hormone-binding globulin and corticosteroid-binding globulin) in humans. Eur J Endocrinol 1995;132:594-8. [Crossref] [PubMed]
- Sheckter CB, Matsumoto AM, Bremner WJ. Testosterone administration inhibits gonadotropin secretion by an effect directly on the human pituitary. J Clin Endocrinol Metab 1989;68:397-401. [Crossref] [PubMed]
- Buzi F, Badolato R, Mazza C, et al. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome: time to review diagnostic criteria? J Clin Endocrinol Metab 2003;88:3146-8. [Crossref] [PubMed]
- Hayes FJ, Seminara SB, Crowley WF Jr. Hypogonadotrophic hypogonadism. Endocrinol Metab Clin North Am 1998;27:739-63. [Crossref] [PubMed]
- Bhasin S, Enzlin P, Coviello A, et al. Sexual dysfunction in men and women with endocrine disorders. Lancet 2007;369:597-611. [Crossref] [PubMed]
- Quinton R, Conway GS, Jacobs HS, et al. Adult onset idiopathic hypogonadotrophic hypogonadism may be overdiagnosed. BMJ 1998;317:600-1. [Crossref] [PubMed]
- Citron JT, Ettinger B, Rubinoff H, et al. Prevalence of hypothalamic-pituitary imaging abnormalities in impotent men with secondary hypogonadism. J Urol 1996;155:529-33. [Crossref] [PubMed]
- Delavierre D, Girard P, Peneau M, et al. Should plasma prolactin assay be routinely performed in the assessment of erectile dysfunction? Report of a series of 445 patients. Review of the literature. Prog Urol 1999;9:1097-101. [PubMed]
- Snyder PJ, Rudenstein RS, Gardner DF, et al. Repetitive infusion of gonadotropin-releasing hormone distinguishes hypothalamic from pituitary hypogonadism. J Clin Endocrinol Metab 1979;48:864-8. [Crossref] [PubMed]
- Begon S, Leyendecker G, Fahlbusch R, et al. Pulsatile administration of gonadotrophin releasing hormone as a diagnostic tool to distinguish hypothalamic from pituitary hypogonadism following neurosurgery. Hum Reprod 1993;8 Suppl 2:200-3. [Crossref] [PubMed]
- Yoshimoto Y, Moridera K, Imura H. Restoration of normal pituitary gonadotropin reserve by administration of luteinizing-hormone-releasing hormone in patients with hypogonadotropic hypogonadism. N Engl J Med 1975;292:242-5. [Crossref] [PubMed]
- Nachtigall LB, Boepple PA, Pralong FP, et al. Adult-onset idiopathic hypogonadotropic hypogonadism--a treatable form of male infertility. N Engl J Med 1997;336:410-5. [Crossref] [PubMed]
- Büchter D, Behre HM, Kliesch S, et al. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol 1998;139:298-303. [Crossref] [PubMed]
- Mao J, Xu H, Wang X, et al. Congenital combined pituitary hormone deficiency patients have better responses to gonadotrophin-induced spermatogenesis than idiopathic hypogonadotropic hypogonadism patients. Hum Reprod 2015;30:2031-7. [Crossref] [PubMed]
- Allegra A, Capra M, Cuccia L, et al. Hypogonadism in beta-thalassemic adolescents: a characteristic pituitary-gonadal impairment. The ineffectiveness of long-term iron chelation therapy. Gynecol Endocrinol 1990;4:181-91. [Crossref] [PubMed]
- Vermeulen A, Andó S, Verdonck L. Prolactinomas, testosterone-binding globulin, and androgen metabolism. J Clin Endocrinol Metab 1982;54:409-12. [Crossref] [PubMed]
- Buvat J, Lemaire A, Buvat-Herbaut M, et al. Hyperprolactinemia and sexual function in men. Horm Res 1985;22:196-203. [Crossref] [PubMed]
- Bancroft J, O’Caroll R, Neilly A, et al. The effects of bromocriptine on the sexual behavior of hyperprolactinemic man: A controlled case-study. Clin Endocrinol (Oxf) 1984;21:131-7. [Crossref] [PubMed]
- Ciccarelli A, Guerra E, De Rosa M, et al. PRL secreting adenomas in male patients. Pituitary 2005;8:39-42. [Crossref] [PubMed]
- Bole-Feysot C, Goffin V, Edery M, et al. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 1998;19:225-68. [Crossref] [PubMed]
- Segal S, Ron M, Laufer N, et al. Prolactin in seminal plasma of infertile men. Arch Androl 1978;1:49-52. [Crossref] [PubMed]
- Sueldo CE, Berger T, Kletzky O, et al. Seminal prolactin concentration and sperm reproductive capacity. Fertil Steril 1985;43:632-5. [Crossref] [PubMed]
- Colao A, Vitale G, Cappabianca P, et al. Outcome of cabergoline treatment in men with prolactinoma: effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysis. J Clin Endocrinol Metab 2004;89:1704-11. [Crossref] [PubMed]
- De Rosa M, Zarrilli S, Vitale G, et al. Six months of treatment with cabergoline restores sexual potency in hyperprolactinemic males: an open longitudinal study monitoring nocturnal penile tumescence. J Clin Endocrinol Metab 2004;89:621-5. [Crossref] [PubMed]
- Lobo RA, Kletzky OA. Normalization of androgen and sex-hormone-binding globulin levels after treatment of hyperprolactinemia. J Clin Endocr Metab 1983;56:562-6. [Crossref] [PubMed]
- Manandhar MS, Thomas JA. Effect of prolactin on the metabolism of androgens by the rat ventral prostate gland in vitro. Invest Urol 1976;14:20-2. [PubMed]
- Dev R, Del Fabbro E, Bruera E. Association between megestrol acetate treatment and symptomatic adrenal insufficiency with hypogonadism in male patients with cancer. Cancer 2007;110:1173-7. [Crossref] [PubMed]
- Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, et al. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer 2004;100:851-8. [Crossref] [PubMed]
- Lambert CP, Sullivan DH, Freeling SA, et al. Effects of testosterone replacement and/or resistance exercise on the composition of megestrol acetate stimulated weight gain in elderly men: a randomized controlled trial. J Clin Endocrinol Metab 2002;87:2100-6. [Crossref] [PubMed]
- Bodenner DL, Medhi M, Evans WJ, et al. Effects of megestrol acetate on pituitary function and end-organ hormone secretion: a post hoc analysis of serum samples from a 12-week study in healthy older men. Am J Geriatr Pharmacother 2005;3:160-7. [Crossref] [PubMed]
- Geller J, Albert J, Yen SS, et al. Medical castration of males with megestrol acetate and small doses of diethylstilbestrol. J Clin Endocrinol Metab 1981;52:576-80. [Crossref] [PubMed]
- MacAdams MR, White RH, Chipps BE. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann Intern Med 1986;104:648-51. [Crossref] [PubMed]
- Luton JP, Thiebolt P, Valcke JC, et al. Reversible gonadotropin deficiency in male Cushing disease. J Clin Endocrinol Metab 1977;45:488-95. [Crossref] [PubMed]
- Marazuela M, Cuerda C, Lucas T, et al. Anterior pituitary function after adrenalectomy in patients with Cushing's syndrome. Postgrad Med J 1993;69:547-51. [Crossref] [PubMed]
- Odagiri E, Yamanaka Y, Ishiwatari N, et al. Studies on pituitary-gonadal function in patients with Cushing's syndrome. Endocrinol Jpn 1988;35:421-7. [Crossref] [PubMed]
- Boccuzzi G, Angeli A, Bisbocci D, et al. Effect of synthetic luteinizing hormone releasing hormone (LH-RH) on the release of gonadotropins in Cushing's disease. J Clin Endocrinol Metab 1975;40:892-5. [Crossref] [PubMed]
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:364-89. [Crossref] [PubMed]
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40. [Crossref] [PubMed]
- Growth Hormone Research Society. Pituitary Society. Biochemical assessment and long-term monitoring in patients with acromegaly: statement from a joint consensus conference of the Growth Hormone Research Society and the Pituitary Society. J Clin Endocrinol Metab 2004;89:3099-102. [Crossref] [PubMed]
- Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism. Thyroid 2014;24:1670-751. [Crossref] [PubMed]
- Bahn RS, Burch HB, Cooper DS, et al. American Thyroid Association, American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract 2011;17:456-520. [Crossref] [PubMed]
- Hall WA, Luciano MG, Doppman JL, et al. Pituitary magnetic reso¬nance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 1994;120:817-20. [Crossref] [PubMed]
- Rhoden EL, Estrada C, Levine L, et al. The value of pituitary magnetic resonance imaging in men with hypogonadism. J Urol 2003;170:795-8. [Crossref] [PubMed]
- Dobs AS, El-Deiry S, Wand G, et al. Central hypogonadism: distinguishing idiopathic low testosterone from pituitary tumors. Endocr Pract 1998;4:355-9. [Crossref] [PubMed]
- Hirsch D, Benbassat C, Toledano Y, et al. Pituitary imaging findings in male patients with hypogonadotrophic hypogonadism. Pituitary 2015;18:494-9. [Crossref] [PubMed]
- Dalvi M, Walker BR, Strachan MW, et al. The prevalence of structural pituitary abnormalities by MRI scanning in men presenting with isolated hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf) 2016;84:858-61. [Crossref] [PubMed]
- Bolu SE, Tasar M, Uçkaya G, et al. Increased abnormal pituitary findings on magnetic resonance in patients with male idiopathic hypogonadotrophic hypogonadism. J Endocrinol Invest 2004;27:1029-33. [Crossref] [PubMed]
- Benbrook D, Pfahl M. A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science 1987;238:788-91. [Crossref] [PubMed]
- Röjdmark S, Berg A, Kallner G. Hypothalamic-pituitary-testicular axis in patients with hyperthyroidism. Horm Res 1988;29:185-90. [Crossref] [PubMed]
- Donnelly P, White C. Testicular dysfunction in men with primary hypothyroidism; reversal of hypogonadotrophic hypogonadism with replacement thyroxine. Clin Endocrinol (Oxf) 2000;52:197-201. [Crossref] [PubMed]
- Jaya Kumar B, Khurana ML, Ammini AC, et al. Reproductive endocrine functions in men with primary hypothyroidism: effect of thyroxine replacement. Horm Res 1990;34:215-8. [Crossref] [PubMed]
- Slag MF, Morley JE, Elson MK, et al. Impotence in medical clinic outpatients. JAMA 1983;249:1736-40. [Crossref] [PubMed]
- Krassas GE, Tziomalos K, Papadopoulou F, et al. Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J Clin Endocrinol Metab 2008;93:1815-9. [Crossref] [PubMed]
- Carani C, Isidori AM, Granata A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab 2005;90:6472-9. [Crossref] [PubMed]
- Veronelli A, Masu A, Ranieri R, et al. Prevalence of erectile dysfunction in thyroid disorders: comparison with control subjects and with obese and diabetic patients. Int J Impot Res 2006;18:111-4. [Crossref] [PubMed]
- Corona G, Petrone L, Mannucci E, et al. Psycho-biological correlates of rapid ejaculation in patients attending an andrologic unit for sexual dysfunctions. Eur Urol 2004;46:615-22. [Crossref] [PubMed]
- Krassas GE, Pontikides N, Deligianni V, et al. A prospective controlled study of the impact of hyperthyroidism on reproductive function in males. J Clin Endocrinol Metab 2002;87:3667-71. [Crossref] [PubMed]