Risk stratification for kidney sparing procedure in upper tract urothelial carcinoma
Introduction
Upper tract urothelial carcinoma (UTUC) is a rare tumour with an incidence of 1 to 2 cases per 100,000 inhabitants. It accounts for 5–10% of all urothelial carcinomas (1,2). Although it shares similar features with urothelial carcinoma of the bladder, UTUC is now considered as a unique entity with specific recommendations (2-4). Radical nephro-ureterectomy (RNU) is still considered the standard treatment for patients with localized UTUC. However, RNU has a significant morbidity and alters renal function. Kidney sparing procedures (KSP) such as segmental ureterectomy and percutaneous or endoscopic ablation have been proposed to preserve renal function while providing similar oncologic results in selected patients (3). KSP were initially limited to imperative indications (i.e., renal insufficiency or solitary functional kidney) but the development of diagnostic URS over the last two decades has dramatically enhanced its role in the management of UTUC (5). Contemporary data now suggest that KSP, especially segmental ureterectomy and endoscopic management, are reasonable and safe treatment options in selected patients with normal and functional contralateral kidneys (3).
Appropriate patient selection is of utmost importance to ensure safety and efficiency of KSP. A risk-adapted approach has been proposed to identify patients that are more likely to benefit from KSP. In “low-risk” tumors, KSP is now recommended as the first treatment option in patients with two functional kidneys. Conversely, in “high risk” tumors, KSP is limited to distal ureteral lesions and imperative cases (6). However, due to the lack of high-level evidence, risk stratification to identify patients with “low-” and “high-” risk tumors who may benefit from KSP remains challenging.
The objective of this review was to provide an overview of preoperative tools that can help risk stratify patients for KSP in UTUC and summarize current guidelines and challenges.
Evidence acquisition
A non-systematic Medline/PubMed literature search was performed using a combination of the terms «upper tract urothelial carcinoma» with different keywords: «kidney sparing surgery», «preoperative models», «risk stratification», «biomarkers» and «prognostic factors». Original articles published between January 2003 and January 2016 were included based on their clinical relevance. Additional informative articles were collected by cross referencing the bibliography of previously selected articles.
Preoperative predictive/prognostic factors for UTUC
Patient related factors
The incidence of UTUC is higher in men than women (1). In a study based on the Surveillance, Epidemiology, and End Results database, female gender was associated with a higher proportion of pT3 and high-grade UTUC and was reported to be an independent predictor of pT3 disease (7). However, these findings were not confirmed in large multicenter studies (8,9). Regarding cancer-specific (CSS) and overall survivals (OS), no significant difference was observed after adjustment for other prognostic factors (7-9).
The role of age as a prognostic factor has been assessed in several multicenter and nationwide retrospective studies, which predominantly showed that advanced patient age is associated with more aggressive disease and worse CSS (10,11). This could be related to changes in tumour biology. However, Chromecki et al. reported that advanced age was no longer associated with CSS after adjustment for Eastern Cooperative oncology Group performance status, underlying the prognostic value of this score compared to age only (12).
The relationship between body mass index and UTUC is still a matter of debate. Ehdaie et al. demonstrated that higher body mass index was associated with aggressive pathological features such as lymphovascular invasion (LVI) and worse outcomes (13). Recently, in a cohort of 236 consecutive Chinese patients treated with RNU, preoperative underweight was identified as an independent predictor of worse recurrence-free survivals (RFS) and CSS (14).
The aforementioned differences with regard to gender, age or body mass index may reflect changes in tumor biology but also heterogeneity regarding health care. For this reason, they should not be considered in treatment decision making.
Further evidence exists regarding the independent predictive value of smoking status in UTUC. Smoking status and duration have been associated with advanced and aggressive disease at RNU and worse oncological outcomes (15). Similarly, a higher risk of recurrence should be considered in patients with specific risk factors for UTUC, such as hereditary nonpolyposis colorectal carcinoma (HNPCC) syndrome, and aristolochic acid or analgesic phenacetin exposure (16).
Tumor related factors
Tumor stage and grade
Cytology, imaging and ureteroscopic biopsy now play a major role to evaluate stage and grade of UTUC, which are the most accurate independent factors of outcomes (17).
Cytology from voided samples or direct washing/exfoliation during endoscopy allows identification of malignant tumor cells that suggest the presence of high-grade/CIS disease. However, cytology still suffers from a lack of reproducibility among cytopathologists while its sensitivity ranges from 43% to 78% with an estimated false negative rate that reaches 50% for low-grade disease (3). However, this lack of performance is balanced by a high specificity for high-grade disease that exceeds 90% with selective ureteral cytology (18).
Flexible ureteroscopy now allows for preoperative visualization of the entire upper urinary tract and tumor biopsy if necessary. Despite several limitations related to the small sample size (19), the biopsy accuracy for tumor grading ranges from 69% to 91%, with a better predictive value for low- than high-grade tumors (3,20). However, the biopsy performance for tumor staging is limited. In a study that included 56 UTUC patients who underwent surgical resection of the tumor with prior biopsy, the stage discrepancy between final RNU pathology and endoscopic biopsy was 38% (21). Nevertheless, biopsy grading may be used to improve tumor staging: 68–100% of grade 1 tumors on biopsies are non-muscle invasive tumors while 62–100% of grade 3 are ≥ pT2 tumors (20). Recently, the impact of cancer detection rate during early repeated flexible ureteroscopy (2nd-look-ureteroscopy within 60 days of the first ureteroscopy) has been recently reported in patients initially treated with endoscopic laser ablation (22). At a median follow-up of 27.6 months, massive tumour (defined as a tumour not completely removable with a kidney sparing approach) RFS rates were 88% and 48% in patients with negative and positive 2nd-look-ureteroscopy, respectively.
Despite the advent of diagnostic URS, pre-operative staging is still largely based on imaging. Multi detector computed tomography urography (MDCT-U) remains the standard technique (23). However, with an accuracy to stage the tumor that ranges from 59% to 88% (24,25); the risk of understaging the tumor with MDCT-U is significant. Nevertheless, the positive predictive value for detecting muscle-invasive features on MDCT-U is high and should be considered as an important information (26). Preliminary reports suggest that multiparametric magnetic resonance imaging (MRI), especially ADC, may be useful in the future to better evaluate tumor stage and grade (27).
Cytology, biopsy grading and imaging should be combined to reach high negative predictive value for muscle invasive disease or higher stage (26). In the same way, positive exfoliated cell cytology increased the accuracy of biopsy grade in determining the presence of advanced-stage UTUC (28,29). The combination of these different information may be useful to overcome their current limitations.
Tumor size, location and multifocality
Both imaging and ureteroscopy provide valuable information on tumor size, location and multifocality. A recent meta-analysis of several studies that assessed the impact of tumor size on oncological outcomes demonstrated that large tumors were associated with a higher risk of recurrence (30). The impact of tumor location (renal pelvis compared to ureter) is still a matter of debate. A recent meta-analysis pooled the results from 17 studies with contradictory conclusions and included more than 12,000 patients. No difference was observed regarding the rates of non-organ confined disease among the different locations (31). However, this and other meta-analyses reported that ureteral lesions were associated with worse RFS and CSS (31,32). Similar findings were reported with tumor multifocality regarding disease progression and cancer-specific mortality (31). Unfortunately, variations regarding the definitions of location, multifocality, and heterogeneity weaken any conclusion.
Tumor architecture and other pathological features on biopsies
Ureteroscopy can help to appreciate tumor architecture (papillary vs. sessile) and biopsies can provide information regarding concomitant carcinoma in situ (CIS), variant histology, LVI or tumor necrosis. It is well-established that most of these parameters from RNU specimen are associated with tumor aggressiveness (33). However, the reliability of such pathological features from small biopsy samples remains to be proven.
Hydronephrosis
Several studies explored the ability of preoperative hydronephrosis to predict clinicopathological features and outcomes in patients with UTUC. Most of these studies showed that hydronephrosis can foresee advanced pathological T stage (T3 or greater), LVI and high-grade disease (34,35). Cho et al. demonstrated that hydronephrosis could also predict worse outcomes in patient with ureteral tumors (36).
Molecular biomarkers
Pathological prognostic factors such as stage, grade, tumor location, or LVI provide important prognostic information but are not very reliable before extirpative surgery. During the last decade, several tissue, blood, genetic or urinary biomarkers have been proposed to help establishing the prognosis of UTUC. One of the main challenges in UTUC is the integration of these biomarkers into clinical practice, to help risk-stratify the disease in order to identify patients who may safely benefit from KSP.
Genetic and tissue-based biomarkers
Microsatellite instability (MSI) has been identified in many somatic cancers, especially those associated with hereditary HNPCC syndrome. Rouprêt et al. demonstrated that MSI positive status was associated with better outcomes in T2-T3N0M0 UTUC patients (37). Epigenetic changes are common events in cancer and DNA methylation is usually responsible for the repression of gene transcription. In a cohort of 280 UTUC patients, Catto et al. showed that hypermethylation was associated with disease progression and confirmed its role as an independent predictor of progression (38). Monteiro-Reis et al. also demonstrated that low methylation of VIM promoter predicted worse CSS (39). Conversely, in a panel of different genes, the methylation of promoters predicted higher T stage, higher grade, LN metastases, bladder recurrence and worse CSS in a large cohort of 687 UTUC patients (40). Fibroblast growth factor receptor 3 (FGFR3) and TP53 mutations are the most frequent somatic mutations observed in urothelial carcinoma. FGFR3 mutations occur in 50% of all primary bladder tumours and are associated with low stage and grade tumors as well as a good prognosis. van Oers et al. confirmed that in UTUC these mutations were also associated with lower stage and better survival in patients with invasive tumors (41). Finally, Izquierdo et al. examined the role of microRNA (Mir) in UTUC and showed that differential expression patterns of Mir-31 and Mir-149 were associated with a higher probability of tumour recurrence and cancer-specific mortality (42).
Collaborative efforts have led to the identification of several tissue-based biomarkers in UTUC. PI3K, Cyclin D, and Ki-67 have been reported as promising prognostic markers of UTUC in large multicentric studies (17). Krabbe et al. showed in a cohort of 475 patients treated with RNU that Ki-67 expression was associated with adverse pathological features and independently predicted RFS and CSS in high-grade tumors (43). Similar findings with PI3K and Cyclin D, two mTOR biomarkers, were observed in a cohort of 620 patients who underwent RNU or partial ureterectomy (44). As these biomarkers were assessed after surgical resection of the tumors, their prognostic value in the pre-operative setting with small tissue samples from ureteroscopic diagnostic biopsies remains to be proven.
Blood biomarkers
Several blood-based biomarkers have been investigated as prognostic factors in UTUC. In a cohort of 564 patients, Tanaka et al. showed that pre-CRP level was an independent predictor of disease recurrence and cancer specific mortality (45). In a recent meta-analysis, Luo et al. confirmed that preoperative CRP level was significantly associated with poor prognosis (28). Other blood markers related to systemic inflammation have also been investigated. Preoperative neutrophil to lymphocyte ratio was significantly associated with worse pathological features and was an independent risk factor of disease recurrence and cancer-specific mortality (45). Other easily available blood biomarkers such as platelet count, fibrinogen, low levels of hemoglobin, sodium and albumin have also been identified as independent prognostic markers in UTUC (33). However further validation is needed to confirm these findings in large multicentric studies before one can routinely use these markers in clinical practice.
Urinary biomarkers
The role of urinary cytology has been discussed previously. Fluorescence in situ hybridization (FISH) assay in the urine demonstrated good performance in UTUC detection and is becoming popular. Some studies showed that the combination of urine cytology and FISH test may be useful to improve the detection of both invasive and non-invasive tumors (29). However, the prognostic value of FISH has not been demonstrated yet.
Telomeres are repetitive sequences that cap the terminal ends of eukaryotic chromosomes. During each cell division, telomeres decrease in size and lead to chromosomal instability and cell death. The expression of telomerase reverse transcriptase (TERT) and telomerase activation play a pivotal role in the malignant transformation and progression of cancer. A recent study reported an association between TERT promoter mutations in UTUC (detected in the urine) and the occurrence of distant metastases (46). However, further efforts are necessary to demonstrate its prognostic value.
Predictive tools
Accurate preoperative UTUC characterization regarding staging, grading and prognosis remains challenging because of the limitations of imaging, endoscopy and biopsy. To overcome these hurdles, multi-institutional clinical research groups have developed preoperative predictive models to guide clinical decision-making.
To date, several pre-operative models focus on the prediction of muscle invasive and non-organ confined UTUC (Table 1). Brien et al. first proposed to combine the presence of hydronephrosis, high-grade disease on biopsy and positive urinary cytology to predict advanced UTUC in a cohort of 172 patients (48). If all criteria were negative, the negative predictive value for muscle-invasive or non-organ confined UTUC reached 100%. Conversely, the presence of all criteria was associated with positive predictive values of 89% and 73% regarding muscle-invasive and locally advanced disease, respectively. Margulis et al. demonstrated that grade; architecture and location of the tumor were independently associated with non-organ confined disease and developed a nomogram that included these three variables (47). This latter tool showed an accuracy of 77% for predicting non-organ confined UTUC. In the same way, Favaretto et al. combined preoperative data from imaging (local invasion, hydronephrosis) and endoscopy (tumor grade, tumor location) to predict muscle-invasive and/or locally advanced disease with an accuracy of 70% (26). Recently, the French collaborative group on UTUC showed that the best model to predict non-organ-confined disease included female gender, locally advanced stage and positive cytology (50). Finally, based on a cohort of 683 Chinese patients treated with RNU, a model including gender, grade at biopsy, tumor multifocality and architecture reached a 79% discrimination rate to predict non-organ-confined disease (49).
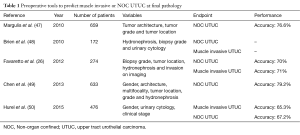
Full table
Few models have been reported to predict RFS and CSS after RNU using pre-operative data. These models may be useful tools to identify tumors with adverse oncologic outcomes that are more likely to receive multimodal approach including radical surgery and maybe, peri-operative chemotherapy. These models are based on imaging, urine cytology, and/or blood markers and support a risk stratification of patients in three groups (51,52). Sakano et al. used a classification based on clinical T stage, urine cytology, and neutrophils blood levels to show that CSS was significantly different among the three groups (51). Fujita et al. based their stratification model on preoperative sodium and hemoglobin levels and found differences in terms of 5-year CSS in the three groups (96.5%, 75.5%, and 47.0%, respectively) (53).
Unfortunately, all these tools are limited by the lack of external validation. Despite promising performance, such validation in independent multicentric cohorts should be done before they can be used in clinical practice.
Guidelines, risk stratification and future challenges
KSP was initially limited to imperative indications such as bilateral disease, renal insufficiency or solitary kidney. In these patients, RNU may lead to dialysis and adverse outcomes compared to KSP. More the half of UTUC patients with two functional kidneys have a glomerular filtration rate <60 mL/min (54). Therefore, a MAG3 renogram may be useful to identify those patients that might experience significant loss of renal function after RNU. Indeed, KSP should always be discussed for patient with a risk of post-operative severe renal insufficiency or dialysis after radical treatment and preferred when this option may provide similar oncologic results compared to radical treatment.
With the development of flexible ureteroscopy and novel instrumentation, KSP have shifted to elective indications to minimize toxicity while preserving oncologic control. Excellent results have been published (3). However, KSP should be offered to well-selected patients with tumors that harbor favorable features and are associated with favorable outcomes. All the aforementioned predictive factors and tools may help identify tumors with muscle invasion or high risk of progression, which may be more likely to benefit from RNU.
The concept of “low-” and “high-” risk tumors has been developed in current guidelines to help clinicians offer KSP to the right patients (2,4). The distinction between low- and high-risk disease relies on tumor multifocality, tumor size, tumor grade on biopsy and cytology, imaging and a history of radical cystectomy. Low risk tumor is defined as a unifocal disease with a tumor size less than 1 cm, a low grade status on cytology or biopsy and no muscle invasion on CT-urography. KSP is now recommended to all compliant patients with such low-risk disease. ICUD consultation suggested that endoscopic treatment could be performed even when tumor size reaches 2 cm provided that cytology is negative, tumor has a papillary architecture and ureteroscopy offers a complete visualization of the tumor (3). Indeed, there is no data in the current literature that support a poorer prognosis for tumours >1 or 2 cm. However, this criterion was added as a surrogate of technical difficulty considering that a complete endoscopic resection is mandatory to ensure oncological safety. Nonetheless, several experts suggested that multifocality and tumor size >1 cm should no longer be an exclusion criteria for laser ablation or segmental ureterectomy (55). In high-risk patients with locally invasive tumors of the distal ureter, segmental ureterectomy with neocystostomy may provide similar oncological outcomes as RNU and therefore may be considered as an option (2).
Accurate identification of low-risk patients remains however challenging and requires a well conducted preoperative work up including cytological, radiographic and endoscopic investigations. Imaging or ureteroscopy may provide valuable information regarding well-known aforementioned predictive and prognostic factors. Unfortunately, even with this exhaustive evaluation, the risk of misclassification of the tumor has been estimated to occur in 25% of the cases (56). Ureteroscopy should provide information regarding location, number, size and aspects of the lesions. It should also assess the feasibility of an endoscopic resection when planned. Multiple biopsies should be done to assess tumor grade while voided cytology, and selective cytology from the upper tract, should document the presence of malignant cells that suggest high-grade tumour or CIS. On the other hand, imaging with MDCT-U is mandatory to identify muscle-invasive and non-organ confined disease.
Despite the different predictive tools that are available to predict muscle-invasive or non-organ confined disease, none are recommended to help physicians and patients in clinical decision making. Indeed, several limitations preclude their widespread use in daily practice. There has been no validation of these tools in external cohorts. In addition, these models only help predict pathological outcomes but do not provide any information regarding patient outcomes after KSP.
Current criteria used to risk-stratify UTUC are still limited and further patient and tumor related factors should be considered for evidence-based counseling. KSP should be considered as a treatment of choice in patients with a high risk of further low risk UTUC during the follow-up. A high risk of UTUC recurrence is predictable in active smokers and patients with UTUC risk factors such as HNPCC syndrome, and aristolochic acid or analgesic phenacetin exposure. Biomarkers that capture the biology of the tumor may help improve risk stratification in UTUC. However, none of them is recommended in current guidelines to help clinical decision making regarding KSP. The vast majority of the studies that assessed the predictive value of biomarkers suffer from their retrospective design and small sample size. Most of the tissue-based biomarkers have been assessed in RNU specimen and their predictive value in small samples from ureteroscopic biopsies still need confirmation. The first study to assess the expression of cell cycle biomarkers both on ureteroscopic biopsies and surgical resections has been recently published. In this cohort of 15 patients, the concordance was 60% (57). Finally, some of these biomarkers may not add any relevant additional information to the current models for clinical decision making. C-index usually assesses the accuracy of the prognostic models. Two recent studies demonstrated that the addition of Ki-67 or a biomarker score only improved pre-operative models by 4% at most (43,44). Further collaborative efforts are necessary to validate current predictive models, identify biomarkers assessable in small tissue samples from endoscopic biopsy and validate their relevance in the pre-operative setting to accurately identify those patients that may benefit from KSP.
Conclusions
KSP is now considered as a safe and efficient alternative to RNU in a risk-based and personalized approach that allows identification of “low-risk” patients currently considered as the best candidates for such management. This risk stratification models rely on patient related factors and tumor characteristics based on imaging, endoscopy and biopsy. However, the lack of high-level evidence based data and current limitations of staging and grading in clinical practice warrants further investigations to improve KSP decision making.
Acknowledgements
None.
Footnote
Conflicts of Interest: Shahrokh F. Shariat owns or co-owns the following patents: methods to determine prognosis after therapy for prostate cancer. Granted 2002-09-06; methods to determine prognosis after therapy for bladder cancer. Granted 2003-06-19; prognostic methods for patients with prostatic disease. Granted 2004-08-05; soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma. Granted 2010-07-20. He is advisory board member of Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanofi, Wolff. He is speaker for Astellas, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, Wolff. Romain Mathieu is consultant for Astellas, Ipsen, Janssen; and he is speaker of Janssen, Sanofi, Novartis, Takeda. R Mathieu—Consultant: Astellas, Ipsen, Janssen; Speaker: Janssen, Sanofi, Novartis, Takeda. The other authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol 2015;68:868-79. [Crossref] [PubMed]
- Mandalapu RS, Remzi M, de Reijke TM, et al. Update of the ICUD-SIU consultation on upper tract urothelial carcinoma 2016: treatment of low-risk upper tract urothelial carcinoma. World J Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Gakis G, Schubert T, Alemozaffar M, et al. Update of the ICUD-SIU consultation on upper tract urothelial carcinoma 2016: treatment of localized high-risk disease. World J Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Audenet F, Traxer O, Yates DR, et al. Potential role of photodynamic techniques combined with new generation flexible ureterorenoscopes and molecular markers for the management of urothelial carcinoma of the upper urinary tract. BJU Int 2012;109:608-13; discussion 613-4. [Crossref] [PubMed]
- Seisen T, Peyronnet B, Dominguez-Escrig JL, et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol 2016. [Epub ahead of print]. [Crossref]
- Lughezzani G, Sun M, Perrotte P, Shariat SF, et al. Gender-related differences in patients with stage I to III upper tract urothelial carcinoma: results from the Surveillance, Epidemiology, and End Results database. Urology 2010;75:321-7. [Crossref] [PubMed]
- Fernández MI, Shariat SF, Margulis V, et al. Evidence-based sex-related outcomes after radical nephroureterectomy for upper tract urothelial carcinoma: results of large multicenter study. Urology 2009;73:142-6. [Crossref] [PubMed]
- Shariat SF, Favaretto RL, Gupta A, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2011;29:481-6. [Crossref] [PubMed]
- Shariat SF, Godoy G, Lotan Y, et al. Advanced patient age is associated with inferior cancer-specific survival after radical nephroureterectomy. BJU Int 2010;105:1672-7. [Crossref] [PubMed]
- Yap SA, Schupp CW, Chamie K, et al. Effect of age on transitional cell carcinoma of the upper urinary tract: presentation, treatment, and outcomes. Urology 2011;78:87-92. [Crossref] [PubMed]
- Chromecki TF, Ehdaie B, Novara G, et al. Chronological age is not an independent predictor of clinical outcomes after radical nephroureterectomy. World J Urol 2011;29:473-80. [Crossref] [PubMed]
- Ehdaie B, Chromecki TF, Lee RK, et al. Obesity adversely impacts disease specific outcomes in patients with upper tract urothelial carcinoma. J Urol 2011;186:66-72. [Crossref] [PubMed]
- Liu JY, Li YH, Liu ZW, et al. Influence of body mass index on oncological outcomes in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Int J Urol 2014;21:136-42. [Crossref] [PubMed]
- Rink M, Xylinas E, Margulis V, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol 2013;63:1082-90. [Crossref] [PubMed]
- Ouzzane A, Rouprêt M, Leon P, et al. Epidemiology and risk factors of upper urinary tract tumors: literature review for the yearly scientific report of the French National Association of Urology. Prog Urol 2014;24:966-76. [Crossref] [PubMed]
- Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol 2012;62:100-14. [Crossref] [PubMed]
- Chen L, He H, Zarka MA, et al. Upper tract urinary cytology to detect upper tract urothelial carcinoma: Using the Johns Hopkins Hospital template and evaluation of its feasibility. Cytojournal 2015;12:17. [PubMed]
- Tavora F, Fajardo DA, Lee TK, et al. Small endoscopic biopsies of the ureter and renal pelvis: pathologic pitfalls. Am J Surg Pathol 2009;33:1540-6. [Crossref] [PubMed]
- Cutress ML, Stewart GD, Zakikhani P, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int 2012;110:614-28. [Crossref] [PubMed]
- Smith AK, Stephenson AJ, Lane BR, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology 2011;78:82-6. [Crossref] [PubMed]
- Villa L, Cloutier J, Letendre J, et al. Early repeated ureteroscopy within 6-8 weeks after a primary endoscopic treatment in patients with upper tract urothelial cell carcinoma: preliminary findings. World J Urol 2016;34:1201-6. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Compérat E, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 2013;63:1059-71. [Crossref] [PubMed]
- Scolieri MJ, Paik ML, Brown SL, et al. Limitations of computed tomography in the preoperative staging of upper tract urothelial carcinoma. Urology 2000;56:930-4. [Crossref] [PubMed]
- Fritz GA, Schoellnast H, Deutschmann HA, et al. Multiphasic multidetector-row CT (MDCT) in detection and staging of transitional cell carcinomas of the upper urinary tract. Eur Radiol 2006;16:1244-52. [Crossref] [PubMed]
- Favaretto RL, Shariat SF, Savage C, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int 2012;109:77-82. [Crossref] [PubMed]
- Akita H, Jinzaki M, Kikuchi E, et al. Preoperative T categorization and prediction of histopathologic grading of urothelial carcinoma in renal pelvis using diffusion-weighted MRI. AJR Am J Roentgenol 2011;197:1130-6. [Crossref] [PubMed]
- Luo Y, Fu SJ, She DL, et al. Preoperative C-reactive protein as a prognostic predictor for upper tract urothelial carcinoma: A systematic review and meta-analysis. Mol Clin Oncol 2015;3:924-928. [PubMed]
- Reynolds JP, Voss JS, Kipp BR, et al. Comparison of urine cytology and fluorescence in situ hybridization in upper urothelial tract samples. Cancer Cytopathol 2014;122:459-67. [Crossref] [PubMed]
- Yuan H, Chen X, Liu L, et al. Risk factors for intravesical recurrence after radical nephroureterectomy for upper tract urothelial carcinoma: a meta-analysis. Urol Oncol 2014;32:989-1002. [Crossref] [PubMed]
- Wu Y, Dong Q, Liu L, et al. The impact of tumor location and multifocality on prognosis for patients with upper tract urothelial carcinoma: a meta-analysis. Sci Rep 2014;4:6361. [Crossref] [PubMed]
- Seisen T, Granger B, Colin P, et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol 2015;67:1122-33. [Crossref] [PubMed]
- Mbeutcha A, Rouprêt M, Kamat AM, et al. Prognostic factors and predictive tools for upper tract urothelial carcinoma: a systematic review. World J Urol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Ito Y, Kikuchi E, Tanaka N, et al. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J Urol 2011;185:1621-6. [Crossref] [PubMed]
- Ng CK, Shariat SF, Lucas SM, et al. Does the presence of hydronephrosis on preoperative axial CT imaging predict worse outcomes for patients undergoing nephroureterectomy for upper-tract urothelial carcinoma? Urol Oncol 2011;29:27-32. [Crossref] [PubMed]
- Cho KS, Hong SJ, Cho NH, et al. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology 2007;70:662-6. [Crossref] [PubMed]
- Rouprêt M, Fromont G, Azzouzi AR, et al. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology 2005;65:1233-7. [Crossref] [PubMed]
- Catto JW, Azzouzi AR, Rehman I, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol 2005;23:2903-10. [Crossref] [PubMed]
- Monteiro-Reis S, Leça L, Almeida M, et al. Accurate detection of upper tract urothelial carcinoma in tissue and urine by means of quantitative GDF15, TMEFF2 and VIM promoter methylation. Eur J Cancer 2014;50:226-33. [Crossref] [PubMed]
- Xiong G, Liu J, Tang Q, et al. Prognostic and predictive value of epigenetic biomarkers and clinical factors in upper tract urothelial carcinoma. Epigenomics 2015;7:733-44. [Crossref] [PubMed]
- van Oers JM, Zwarthoff EC, Rehman I, et al. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur Urol 2009;55:650-7. [Crossref] [PubMed]
- Izquierdo L, Ingelmo-Torres M, Mallofré C, et al. Prognostic value of microRNA expression pattern in upper tract urothelial carcinoma. BJU Int 2014;113:813-21. [Crossref] [PubMed]
- Krabbe LM, Bagrodia A, Haddad AQ, et al. Multi-institutional validation of the predictive value of Ki-67 in patients with high grade urothelial carcinoma of the upper urinary tract. J Urol 2015;193:1486-93. [Crossref] [PubMed]
- Bagrodia A, Krabbe LM, Gayed BA, et al. Evaluation of the prognostic significance of altered mammalian target of rapamycin pathway biomarkers in upper tract urothelial carcinoma. Urology 2014;84:1134-40. [Crossref] [PubMed]
- Tanaka N, Kikuchi E, Shirotake S, et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol 2014;65:227-34. [Crossref] [PubMed]
- Wang K, Liu T, Ge N, et al. TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive castPCR. Oncotarget 2014;5:12428-39. [Crossref] [PubMed]
- Margulis V, Youssef RF, Karakiewicz PI, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol 2010;184:453-8. [Crossref] [PubMed]
- Brien JC, Shariat SF, Herman MP, et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol 2010;184:69-73. [Crossref] [PubMed]
- Chen XP, Xiong GY, Li XS, et al. Predictive factors for worse pathological outcomes of upper tract urothelial carcinoma: experience from a nationwide high-volume centre in China. BJU Int 2013;112:917-24. [PubMed]
- Hurel S, Rouprêt M, Seisen T, et al. Influence of preoperative factors on the oncologic outcome for upper urinary tract urothelial carcinoma after radical nephroureterectomy. World J Urol 2015;33:335-41. [Crossref] [PubMed]
- Sakano S, Matsuyama H, Kamiryo Y, et al. Risk group stratification based on preoperative factors to predict survival after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Ann Surg Oncol 2013;20:4389-96. [Crossref] [PubMed]
- Hashimoto T, Ohno Y, Nakashima J, et al. Clinical significance of preoperative peripheral blood neutrophil count in patients with non-metastatic upper urinary tract carcinoma. World J Urol 2013;31:953-8. [Crossref] [PubMed]
- Fujita K, Uemura M, Yamamoto Y, et al. Preoperative risk stratification for cancer-specific survival of patients with upper urinary tract urothelial carcinoma treated by nephroureterectomy. Int J Clin Oncol 2015;20:156-63. [Crossref] [PubMed]
- Xylinas E, Rink M, Margulis V, et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int 2013;112:453-61. [Crossref] [PubMed]
- Seisen T, Colin P, Rouprêt M. Risk-adapted strategy for the kidney-sparing management of upper tract tumours. Nat Rev Urol 2015;12:155-66. [Crossref] [PubMed]
- Williams SK, Denton KJ, Minervini A, et al. Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol 2008;22:71-6. [Crossref] [PubMed]
- Gayed BA, Bagrodia A, Gaitonde M, et al. Feasibility of obtaining biomarker profiles from endoscopic biopsy specimens in upper tract urothelial carcinoma: preliminary results. Urol Oncol 2015;33:18.e21-6. [Crossref] [PubMed]


