Adult-onset hypogonadism: evaluation and role of testosterone replacement therapy
Brief overview/definition/prevalence
The Sexual Medicine Society of North America defines adult-onset hypogonadism (AOH) as a clinical and biochemical syndrome characterized by a deficiency of testosterone with associated signs and symptoms and a failure of the body to produce an adequate compensatory response (1). AOH can be caused by testicular and/or hypothalamic-pituitary dysfunction, making it clinically distinct from primary and secondary hypogonadism. The previously quoted dichotomous distinction of primary and secondary hypogonadism was seen to be falsely applicable to a large majority of the population of men being treated for AOH. In these men, low testosterone levels are often accompanied by normal or low levels of gonadotropins, indicating physiologic failure at both the testicular and hypothalamic-pituitary levels. When defining the prevalence of AOH, the Sexual Medicine Society of North America examined data from large epidemiologic studies, such as the European Male Ageing Study (2). In this study of 3,369 men between 40 and 79 years of age from eight different European cities, the gonadal status was defined based on normal cut-off values of 300 ng/dL for total testosterone (TT) and 9.4 U/L for luteinizing hormone (LH). Using these aforementioned thresholds, the study identified an overall prevalence of hypogonadism of 13.8%; however, 85.5% of these men were diagnosed with secondary hypogonadism with low testosterone and low or normal LH, a similar presentation seen with AOH. It is important to note that this classification of secondary hypogonadism does not accurately establish the extent to which low testosterone is due to inadequate gonadotropins because many of these men may have a testicular failure component as well. In a study by Corona and Maggi, nearly 90% of men with secondary hypogonadism had no identifiable medical condition that could account for their hypogonadism other than a concomitant metabolic disease (i.e., diabetes mellitus, obesity, or metabolic syndrome), which was seen in over 70% of these men (3). Although the gonadal status is a key component to the clinical entity of AOH, it should be noted that the actual prevalence of symptomatic hypogonadism defined as low testosterone (<300 ng/dL) with at least three signs of symptoms of hypogonadism ranges from 2% to 6%, as identified in the European Male Ageing Study and Boston Area Community Health Survey studies (4).
Clinical presentation/evaluation
AOH is an overlooked and underdiagnosed condition because signs and symptoms can be variable and nonspecific and can present slowly over time (5). As a result, many men ignore their symptoms, and many clinicians fail to diagnose AOH without a high clinical index of suspicion. The most common presenting symptoms are centered on sexual dysfunction, given testosterone’s critical role in sexual response. These symptoms may include reduced sexual desire, reduced nocturnal and morning erections, reduced sex-induced erections, delayed ejaculation, and reduced semen volume (6). The most prevalent clinical symptoms in both older and younger men with hypogonadism are decreased spontaneous erections, low libido, and difficulty maintaining an erection (7). The most prevalent nonsexual symptoms are fatigue, muscle weakness, depressed mood, and increased body fat. Other reported symptoms include excessive irritability, memory difficulties, poor concentration, sleep disturbances, and diminished work performance (2,6,7).
Once a history is taken, a physical exam should be performed to identify any physical signs to help supplement and support a diagnosis of hypogonadism based on a clinical history. Although the clinical examination is usually normal in men with testosterone deficiency (TD), a number of genital abnormalities may suggest the presence of TD, including cryptorchidism, small or soft testes, and varicocele (8). The following parameters should be documented: assessment of general body habitus, any signs of breast development, muscle development, hair growth, inspection of the penis to assess postpubertal development, and palpation of scrotal contents. When evaluating the testes, it is important to note maldescent, evidence of varicocele, size and consistency of the testes and epididymis, and any evidence of scarring from previous inflammation or infection. A digital rectal exam should be discussed for any man over the age of 40 as part of screening for prostate adenocarcinoma before any treatment for TD is recommended.
Laboratory testing
When clinical suspicion indicates the need to evaluate the patient for hypogonadism, biochemical testing should be pursued. Patients presenting with acute or subacute symptoms may have a low testosterone level and should have their evaluation deferred. When testing for testosterone levels, morning blood testing is recommended due to diurnal variation; although, this may not be necessary in men over the age of 40 due to the minimal observed diurnal changes in that population (9). There is no universally accepted serum testosterone concentration indicative of low testosterone. In most clinical trials, a cut-off value of 300 ng/dL is used to define hypogonadism. However, multiple academic societies, including the International Society of Andrology, the International Society for Study of the Aging Male, the European Association of Urology, the European Association of Andrology, and the American Society of Andrology do not recommend therapy for TT levels >350 ng/dL but recommend testosterone replacement therapy (TRT) for TT levels below 230 ng/dL when associated symptoms are noted. For levels between 230–350 ng/dL, a repeat TT with sex hormone binding globulin (SHBG) is recommend for the calculation of free or bioavailable testosterone.
Since the interpretation of TT concentrations can be confounded by SHBG concentrations, it is often useful to obtain free testosterone levels in conjunction with TT. Free testosterone can be measured directly by radioimmunoassay (RIA) or calculated from TT and SHBG concentrations. Reference ranges for free testosterone provided by laboratory reports should be used cautiously, with the knowledge that they vary widely from laboratory to laboratory and are not clinically based. A number of authorities have suggested the use of calculated free testosterone values less than 80–100 pg/mL (10,11).
If initial testing is indicative of hypogonadism, free and TT should be repeated and testing of LH, thyroid-stimulating hormone, and prolactin can be ordered to help delineate causes of hypogonadism before commencement of TRT. Hemoglobin/hematocrit (Hct) and prostate-specific antigen (PSA) levels in men over 40 years of age should also be considered to provide baseline measurements for future long-term monitoring.
Treatment types
Once AOH is accurately diagnosed, the physician must discuss all treatment options for TRT, including the option of no treatment. When considering TRT, the goals of therapy should include restoration of testosterone levels to the mid-normal range, approximation of endogenous production, avoidance or reduction of significant adverse effects, and alleviation of the associated the signs and symptoms of AOH. Overall, there is evidence suggesting improvement in physical condition, sexual libido, glucose control, lipid metabolism, mood, and cognition (Table 1). These benefits may differ in older men as exemplified in a recent trial by Snyder et al. (12). The effects of restoring testosterone levels in hypogonadal men aged 65 years and older over the course of 1 year were evaluated, and benefits were noted in sexual function, mood, and depressive symptoms; although, no change was noted in vitality or walking distance. These are the benefits often sought by men seeking treatment for hypogonadism and should be thoroughly discussed prior to the initiation of therapy in an effort to help manage long-term expectations of TRT.

Full table
When considering TRT, there are multiple options between various testosterone preparations that differ in their ease of use, route of delivery, and cost. The physician should educate the patient on the differences of each of the preparations in order for the patient to best understand the route of therapy most beneficial for their specific circumstance. The first question to answer when initially counseling a patient on TRT should discuss their desire for future fertility. Exogenous testosterone supplementation results in decreased gonadotropic secretion at 3–4 months by feedback inhibition at the level of the hypothalamus and pituitary gland. This feedback inhibition leads to a suppression of follicle stimulating hormone (FSH) and, ultimately, a decrease in spermatogenesis (13). Patients with low testosterone who desire fertility may be treated with agents that avoid the feedback suppression of FSH, such as human chorionic gonadotropin, aromatase inhibitors, or selective estrogen receptor modulators (SERMS). Human chorionic gonadotropin is a gonadotropin analog that can stimulate the release of LH and FSH from the pituitary gland, ultimately increasing testosterone levels, while avoiding concomitant suppression of spermatogenesis. Aromatase inhibitors have also been used as an alternative to TRT for AOH. They work by reducing peripheral conversion of testosterone to estradiol 2 (E2) via enzymatic inhibition of aromatase (14). This medication increases testosterone and reduces E2, altering the testosterone:E2 ratio. For patients with hypogonadism, several trials have demonstrated significant improvements in testosterone over placebo (14-16). Given its effect on E2, concern developed regarding bone health and bone mineral density, and data remains conflicted over the long-term effect on bone strength (16,17). The last medication category, the one used most commonly, is SERMs. This category of medications is commonly used off-label for the treatment of symptomatic hypogonadism and subfertility or infertility. As partial estrogen-blocking agents, these medications decrease negative feedback on the pituitary gland and increase secretion of FSH and LH with resultant increased testosterone production. One recently conducted trial suggests equivalency of SERMs with topical testosterone gel in achieving physiologic testosterone levels, with improved or maintained sperm counts (18).
If fertility is not a concern, testosterone can be directly administered for supplementation and treatment of AOH. There are a variety of formulations currently used (Table 2) (19). The most widely used testosterone preparations in the United States are transdermal and injectable preparations because of their ease of use (transdermal) and relatively low cost (injectable). We will review testosterone supplementation according to the form by which it is administered.
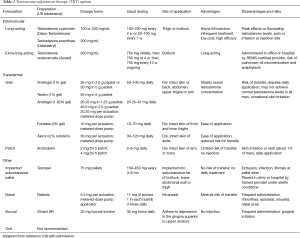
Full table
Oral
Oral testosterone undecanoate undergoes first-pass metabolism and is inactivated in the liver. An oral preparation was created to bypass first-pass metabolism with the methylation at the 17α. Significant hepatotoxic adverse effects have been noted long-term with this modality, and as such, its use is not recommended (20). Oral testosterone undecanoate is not currently available in the United States. Another preparation, oral testosterone undecanoate in castor oil, has been developed and is available in Europe and Canada. This preparation avoids first-pass metabolism because it is preferentially absorbed into the lymphatic system, and as a result, there are fewer hepatic adverse effects. This formulation needs to be taken 2 to 4 times daily with a normal meal, but without adequate dietary fat content, absorption may be incomplete and testosterone levels may not equilibrate.
Buccal
The testosterone buccal system (Striant) is the only available oral testosterone therapy in the United States. Buccal systems are applied every 12 hours to the upper gum, overlying the incisor tooth, with patches alternating between the left and right sides (21). Administration provides a steady delivery of testosterone, which is maintained in physiologic ranges. Two noninferiority trials comparing Striant to either a testosterone transdermal system (Androderm) or testosterone gel (Androgel) demonstrated equivalent physiologic testosterone levels (22-24). Safety and tolerability data from two open-label phase III trials demonstrated a 12% rate of discontinuation over a 2-year period due to adverse events, most commonly altered taste and gum irritation.
Transdermal
Multiple transdermal systems of testosterone delivery are currently available with similar pharmacokinetic and adverse event profiles. Although the sites of delivery vary between formulations, all therapies achieve normal physiologic concentrations of testosterone in over 75% of patients, with slight differences in the rates and peak levels of testosterone achieved (25-29). Dose adjustment is important because transdermal absorption varies between men, and may vary in an individual over time, depending on long-term skin changes at administration sites (20). Skin irritation with blisters is more common with patches compared to gels. Patients undergoing transdermal testosterone supplementation should be cautioned to the potential for direct transference to others, particularly to women and children. As direct skin-to-skin contact is required for transference, this may be avoided by placement of clothing over the administered site and thorough hand washing following topical application.
Injections
Injection therapies with testosterone provide an alternative method for testosterone supplementation. Deep intramuscular injections are performed in a home or office setting every 1 to 4 weeks in the gluteal or quadricep areas. A characteristic of injectable testosterone is the rapid rise to supraphysiologic levels of testosterone within 1 to 2 days of administration, with a gradual decline into the hypogonadal range at the end of the dosing interval (20). Testosterone enanthate 200 mg administered intramuscularly every 2 weeks achieves normal physiologic testosterone levels for 72% of the treatment interval compared to 82% with the testosterone transdermal system (30). Although costs vary by region and insurance plan, in general, intramuscular therapies are currently the least expensive alternative for testosterone supplementation. Adverse events with injectable testosterone include local pain and higher levels of polycythemia secondary to the supraphysiologic surge of testosterone associated with the injections (20).
Pellets
A long-lasting option for testosterone supplementation is subcutaneous testosterone pellets inserted into the lateral buttock or lower abdomen every 3 to 4 months. Different pellet presentations are available around the world. The procedure involves local anesthetic, with the pellets inserted into subcutaneous fat with a small trocar (20). Subcutaneous testosterone pellet insertion achieves peak testosterone levels approximately 12 hours following insertion, with a half-life of approximately 71 days. The total and duration of physiologic testosterone levels vary based on the number of pellets inserted and the patient’s body mass index. Patients with elevated body mass indices achieve lower peak concentrations and may require a larger number of pellets compared to men in the low or normal body mass index range (31-34). Common adverse events associated with testosterone pellet administration include, local pain, erythema, pellet extrusion, and ecchymosis (34).
Monitoring
Monitoring for treatment efficacy and possible adverse events should be based on the Endocrine Society’s guidelines for monitoring patients on TRT (35). Once a decision is made to proceed with TRT, follow-up should be set up for 3 to 6 months, at which time symptoms can be assessed, testosterone levels can be rechecked, and monitoring can be continued for weight, Hct, and PSA. Should TT remain <400 ng/dL, consideration to increase dosing may be pursued. If Hct >54%, TRT should be stopped until it returns to a normal level, and TRT may be reinitiated at a lower dose. In men older than 40 years with a baseline PSA >0.6 ng/mL, one should perform a PSA and digital rectal exam before TRT and at intervals of 3 and 6 months once TRT is initiated. If PSA remains stable, follow-up can continue annually thereafter. If the patient has tolerated TRT well with no laboratory abnormalities, follow-up can continue annually. After 1 to 2 years of TRT in men with osteoporosis or history of low trauma fracture, a bone mineral density test should be pursued. If no improvement is noted after 3 to 6 months of TRT, one should consider other causes of the initial presenting symptoms.
Risks of TRT
Cardiovascular
Hypogonadism and low testosterone levels have been associated with an increased risk of cardiovascular disease (CVD) (36). In this meta-analysis, lower testosterone was correlated with increased risk of CVD and cardiovascular mortality. However, this research did not definitively determine whether low testosterone is simply associated with cardiovascular risk or whether it is an actual causative and potentially modifiable factor. Several analyses have raised concern regarding TRT and increased CVD risk (37-40). Given this concern, the Food and Drug Administration recently required manufacturers of testosterone products to modify the labeling of their product to include a warning regarding potentially increased risks of cardiovascular events (CVE) in the setting of TRT. The debate over the use of TRT and the associated cardiovascular risks remains undecided because no adequately designed, randomized controlled trials have been performed with cardiovascular endpoints. In the largest meta-analysis on the subject of CVE and TRT in 2014, Corona et al. (41) reviewed 75 articles with a combined 5,464 patients over a mean duration of 34 weeks. In this study, no evidence for a causal relationship between TRT and CVE was established. Even at-risk groups, such as elderly men, those with prior CVE, and frail men, did not experience higher rates of CVE, and those with metabolic diseases who received TRT experienced lower rates of CVE compared to placebo. Calof et al. (42) examined 19 placebo-controlled TRT trials and reported no increased risk of CVE in the TRT population compared to control patients. In addition, a large meta-analysis of 51 studies, ranging in methodologic quality from low to medium, found no increased risk of CVE or all-cause mortality associated with TRT (43). Given these conflicting findings, the need for an unambiguous conclusion regarding TRT and CVE remains of paramount importance for the community of providers seeking to best manage and counsel patients with AOH.
Prostate cancer
No adequately designed or appropriately powered study has been conducted to date to assess prostate cancer related risks of TRT. The detrimental effect of testosterone in locally advanced or metastatic prostate cancer has been well established, with early studies demonstrating significant progression of disease following exogenous testosterone administration (44,45). Hsing (46) noted no difference in the incidence of prostate cancer in patients undergoing TRT compared to the general population. Two meta-analyses of placebo-controlled TRT studies revealed no increased risk of the development of prostate cancer for patients undergoing TRT (42,43). As such, the available evidence suggests it is safe to administer testosterone in the setting of AOH without increasing an individual’s long-term risk of prostate cancer.
Several retrospective studies have studied TRT in patients who have undergone definitive therapy for their prostate cancer and have demonstrated no increased risk of prostate cancer recurrence (47-50). These studies have significant limitations given their retrospective nature, and larger randomized controlled trials are needed to evaluate TRT in patients diagnosed with prostate cancer. Current evidence recommends against TRT in the setting of untreated prostate cancer but permits administration at a prudent interval following successful definitive local therapy with no evidence of recurrence.
Lower urinary tract symptoms (LUTS)
Although androgens are thought to play a large role in prostate development, no difference in prostatic androgens has been noted in men with and without benign prostatic hyperplasia (BPH) (51). Many physicians may be concerned about TRT in the setting of BPH; however, multiples studies have demonstrated either no change or improved parameters of voiding and LUTS in patients with BPH undergoing TRT, and thus, BPH should not be a contraindication to TRT in the setting of AOH (42,43,52-57).
Polycythemia
Polycythemia (erythrocytosis) is a common adverse event associated with TRT, that is both dose and serum-level dependent (42,43,58). The overall effect noted varies by dose and patient age, but the risk of an increase in Hct >50% has been noted to be 3 to 4 times higher in patients receiving TRT compared to controls (42,43). The initial rise in hemoglobin and Hct is seen in the first 5 to 6 months, with a decline noted 3 to 12 months after TRT discontinuation (59-62). Although it has been hypothesized that enhance blood viscosity may be a risk for CVE, a causal relationship for TRT and its related erythrocytosis with CVE and mortality have not been well-defined through current studies, as described noted. As such, the Endocrine Society’s Clinical Practice Guidelines are used to guide clinical management of TRT-related polycythemia and state that Hct values >54% warrant discontinuation of TRT until further assessment (35). In cases of extremely elevated or persistent polycythemia, therapeutic phlebotomy has been described as a management option (63).
Acknowledgements
None.
Footnote
Conflicts of Interest: GA Broderick is the Consultant, Abbvie. The other author has no conflicts of interest to declare.
References
- Khera M, Broderick GA, Carson CC 3rd, et al. Adult-onset hypogonadism. Mayo Clin Proc 2016;91:908-26. [Crossref] [PubMed]
- Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: Evidence from the European Male Aging Study. J Clin Endocrinol Metab 2010;95:1810-8. [Crossref] [PubMed]
- Corona G, Maggi M. Perspective: Regulatory agencies’ changes to testosterone product labeling. J Sex Med 2015;12:1690-3. [Crossref] [PubMed]
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241-7. [Crossref] [PubMed]
- Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting P. Int J Clin Pract 2010;64:682-96. [Crossref] [PubMed]
- Buvat J, Maggi M, Guay A, et al. Testosterone deficiency in men: Systematic review and standard operating procedures for diagnosis and treatment. J Sex Med 2013;10:245-84. [Crossref] [PubMed]
- Rosen RC, Araujo AB, Connor MK, et al. The NERI hypogonadism screener: Psychometric validation in male patients and controls. Clin Endocrinol (Oxf) 2011;74:248-56. [Crossref] [PubMed]
- Tanrikut C, Goldstein M, Rosoff JS, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int 2011;108:1480-4. [Crossref] [PubMed]
- Crawford ED, Bargawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int 2007;100:509-13. [Crossref] [PubMed]
- Traish AM, Miner MM, Morgentaler A, et al. Testosterone deficiency. Am J Med 2011;124:578-87. [Crossref] [PubMed]
- Morgentaler A. Commentary: Guideline for male testosterone therapy: a clinician's perspective. J Clin Endocrinol Metab 2007;92:416-7. [Crossref] [PubMed]
- Snyder PJ, Bhasin S, Cunnigham GR, et al. Effects of testosterone treatment in older men. N Engl J Med 2016;374:611-24. [Crossref] [PubMed]
- Anderson RA, Wu FC. Comparison between testosterone enanthate-induced azoospermia and oligozoospermia in a male contraceptive study. II. Pharmacokinetics and pharmacodynamics of once weekly administration of testosterone enanthate. J Clin Endocrinol Metab 1996;81:896-901. [PubMed]
- Burnett-Bowie SA, Roupenian KC, Dere ME, et al. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2009;70:116-23. [Crossref] [PubMed]
- Leder BZ, Rohrer JL, Rubin SD, et al. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels J Clin Endocrinol Metab 2004;89:1174-80. [Crossref] [PubMed]
- Burnett-Bowie SA, McKay EA, Lee H, et al. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab 2009;94:4785-92. [Crossref] [PubMed]
- Leder BZ, Finkelstein JS. Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporos Int 2005;16:1487-94. [Crossref] [PubMed]
- Kaminetsky J, Werner M, Fontenot G, et al. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med 2013;10:1628-35. [Crossref] [PubMed]
- Berkseth KE, Thirumalai A, Amory JK. Pharmacologic therapy in men's health: Hypogonadism, erectile dysfunction, and benign prostatic hyperplasia. Med Clin North Am 2016;100:791-805. [Crossref] [PubMed]
- Dean JD, McMahon CG, Guay AT, et al. The International Society for Sexual Medicine's process of care for the assessment and management of testosterone deficiency in adult men. J Sex Med 2015;12:1660-86. [Crossref] [PubMed]
- Korbonits M, Kipnes M, Grossman AB. Striant SR: a novel, effective and convenient testosterone therapy for male hypogonadism. Int J Clin Pract 2004;58:1073-80. [Crossref] [PubMed]
- Dobs AS, Matsumoto AM, Wang C, et al. Short-term pharmacokinetic comparison of a novel testosterone buccal system and a testosterone gel in testosterone deficient men. Curr Med Res Opin 2004;20:729-38. [Crossref] [PubMed]
- Columbia Laboratories Inc. Data on file: a combined report of two open-label phase III, multicenter studies of COL 1621, The long-term safety, tolerability and efficacy in testosterone-deficient patients. 2004.
- Wang C, Swerdloff R, Kipnes M, et al. New testosterone buccal system (Striant) delivers physiological testosterone levels: Pharmacokinetics study in hypogonadal men. J Clin Endocrinol Metab 2004;89:3821-9. [Crossref] [PubMed]
- Mazer N, Bell D, Wu J, et al. Comparison of the steady-state pharmacokinetics, metabolism, and variability of a transdermal testosterone patch versus a transdermal testosterone gel in hypogonadal men. J Sex Med 2005;2:213-26. [Crossref] [PubMed]
- Wang C, Ilani N, Arver S, et al. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen-deficient men. Clin. Endocrinol (Oxf) 2011;75:836-43. [Crossref] [PubMed]
- Kaufman JM, Miller MG, Garwin JL, et al. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J Sex Med 2011;8:2079-89. [Crossref] [PubMed]
- McNicholas TA, Dean JD, Mulder H, et al. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int 2003;91:69-74. [Crossref] [PubMed]
- Dobs AS, McGettigan J, Norwood P, et al. A novel testosterone 2% gel for the treatment of hypogonadal males. J Androl 2012;33:601-7. [Crossref] [PubMed]
- Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999;84:3469-78. [PubMed]
- Jockenhövel F, Vogel E, Kreutzer M, et al. Pharmacokinetics and pharmacodynamics of subcutaneous testosterone implants in hypogonadal men. Clin Endocrinol (Oxf) 1996;45:61-71. [Crossref] [PubMed]
- Pastuszak AW, Mittakanti H, Liu JS, et al. Pharmacokinetic evaluation and dosing of subcutaneous testosterone pellets. J Androl 2012;33:927-37. [Crossref] [PubMed]
- Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab 1990;71:216-22. [Crossref] [PubMed]
- Kaminetsky JC, Moclair B, Hemani M, et al. A phase IV prospective evaluation of the safety and efficacy of extended release testosterone pellets for the treatment of male hypogonadism. J Sex Med 2011;8:1186-96. [Crossref] [PubMed]
- Bhasin S, Cunnignham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guidline. J Clin Endocrinol Metab 2010;95:2536-59. [Crossref] [PubMed]
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011;165:687-701. [Crossref] [PubMed]
- Vigen R, O'Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013;310:1829-36. [Crossref] [PubMed]
- Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5alpha-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA 2012;307:931-9. [Crossref] [PubMed]
- Tan WS, Low WY, Ng CJ, et al. Efficacy and safety of long-acting intramuscular testosterone undecanoate in aging men: a randomised controlled study. BJU Int 2013;111:1130-40. [Crossref] [PubMed]
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. New Engl J Med 2010;363:109-22. [Crossref] [PubMed]
- Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf 2014;13:1327-51. [Crossref] [PubMed]
- Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 2005;60:1451-7. [Crossref] [PubMed]
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010;95:2560-75. [Crossref] [PubMed]
- Fowler JE Jr, Whitmore WF Jr. The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J Urol 1981;126:372-5. [PubMed]
- Fowler JE Jr, Whitmore WF Jr. Considerations for the use of testosterone with systemic chemotherapy in prostatic cancer. Cancer 1982;49:1373-7. [Crossref] [PubMed]
- Hsing AW. Hormones and prostate cancer: what's next? Epidemiol Rev 2001;23:42-58. [Crossref] [PubMed]
- Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol 2013;190:639-44. [Crossref] [PubMed]
- Pastuszak AW, Pearlman AM, Godoy G, et al. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res 2013;25:24-8. [Crossref] [PubMed]
- Kaufman JM, Graydon RJ. Androgen replacement after curative radical prostatectomy for prostate cancer in hypogonadal men. J Urol 2004;172:920-2. [Crossref] [PubMed]
- Morgentaler A. Two years of testosterone therapy associated with decline in prostate-specific antigen in a man with untreated prostate cancer. J Sex Med 2009;6:574-7. [Crossref] [PubMed]
- van der Sluis TM, Vis AN, van Moorselaar RJ, et al. Intraprostatic testosterone and dihydrotestosterone, part I: concentrations and methods of determination in men with benign prostatic hyperplasia and prostate cancer. BJU Int 2012;109:176-82. [Crossref] [PubMed]
- Pechersky AV, Mazurov VI, Semiglazov VF, et al. Androgen administration in middle-aged and ageing men: Eeffects of oral testosterone undecanoate on dihydrotestosterone, oestradiol and prostate volume. Int J Androl 2002;25:119-25. [Crossref] [PubMed]
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male 2010;13:242-6. [Crossref] [PubMed]
- Francomano D, Ilacqua A, Bruzziches R, et al. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology 2014;83:167-73. [Crossref] [PubMed]
- Haider A, Gooren LJ, Padungtod P, et al. Concurrent improvement of the metabolic syndrome and lower urinary tract symptoms upon normalisation of plasma testosterone levels in hypogonadal elderly men. Andrologia 2009;41:7-13. [Crossref] [PubMed]
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male 2011;14:53-8. [Crossref] [PubMed]
- Pearl JA, Berhanu D, Francois N, et al. Testosterone supplementation does not worsen lower urinary tract symptoms. J Urol 2013;190:1828-33. [Crossref] [PubMed]
- Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 1992;75:1092-8. [PubMed]
- Delev DP, Davcheva DP, Kostadinov ID, et al. Effect of testosterone propionate on erythropoiesis after experimental orchiectomy. Folia Med (Plovdiv) 2013;55:51-7. [Crossref] [PubMed]
- Ip FF, di Pierro I, Brown R, et al. Trough serum testosterone predicts the development of polycythemia in hypogonadal men treated for up to 21 years with subcutaneous testosterone pellets. Eur J Endocrinol 2010;162:385-90. [Crossref] [PubMed]
- Swerdloff RS, Wang C. Three-year follow-up of androgen treatment in hypogonadal men: preliminary report with testosterone gel. Aging Male 2003;6:207-11. [Crossref] [PubMed]
- Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004;89:2085-98. [Crossref] [PubMed]
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol 2008;159:507-14. [Crossref] [PubMed]


