A case series of the safety and efficacy of testosterone replacement therapy in renal failure and kidney transplant patients
Introduction
Since its discovery in the 1930s, testosterone (T) has become a well-established treatment modality for patients with hypogonadism and/or sexual dysfunction. The use of testosterone replacement therapy (TRT) has been on the rise over the past decade. A 3-fold increase in prescription rates was noted in 2011 (1), which corresponded to a 1.6 billion dollar worth of sales (2). This upsurge extends from the many favorable effects T has on various body systems. In addition to its ability to augment sexual function, T is known to enhance physical strength, well-being, mood, bone health, and metabolism. Nevertheless, in few circumstances such as patients with chronic kidney disease (CKD), the use of TRT is still cautiously practiced.
Renal failure, dialysis and transplantation are all known to negatively affect serum T levels. A state of hypothalamo-pituitary-gonadal dysfunction is often encountered in patients with CKD, with a severity that is proportional to the degree of renal impairment (3). Approximately two-thirds of men on haemodialysis (HD) have serum T levels in the hypogonadal range (4). A state of hypogonadism is found to persist in up to 25% of renal transplant recipients even after normalization of their renal function with consequences heightened by the use of immunosuppressive medications (4). Although the nature of the renal disease may indeed overshadow its endocrine and sexual consequences, an understanding of such influence is integral to provide a satisfactory and complete patient care.
Very few studies of confounding results have evaluated TRT in patients with renal disease and mainly focusing on HD patients. Brockenbrough et al. failed to find any improvement in serum T levels of 20 HD patients after 6 months of treatment with topical T (5). Conversely, Singh et al. did convey a significant response to topical T therapy in a same patient subset (6). In terms of symptoms, one study documented improvements in areas of erectile function and measures of sexual satisfaction in nine HD patients treated with topical T (7). Depot T injections, on the other hand, failed to significantly restore the sexual function of 27 HD patients (8). This study is aimed to report our experience with TRT in HD and transplant patients hoping to provide further insight into this inadequately investigated subject.
Methods
This is a retrospective study conducted at the Glickman Urological and Kidney Institute in Cleveland Clinic, Ohio, United States. The institute’s IRB approved Men’s Health Registry was reviewed looking for patients who received TRT. The inclusion criteria were patients with a history of end stage renal disease treated with either HD or renal transplantation. Identified records were evaluated for data collection. The collected data included patients’ demographics, clinical presentation, medical history, type and duration of TRT, symptomatic response to treatment and initial and follow-up laboratory investigations.
Patients received T in the form of transdermal patches (2–6 mg), gel (5 mg) or implanted pellets (75 mg/pellet). The transdermal T was applied to the back, abdomen, upper arm, or upper thigh once a day. The pellets were implanted subcutaneously every 3–4 months to alternate buttocks. Investigations consisted of serum T (reference range, 260–1,000 ng/dL), prostate specific antigen (PSA, reference range, 0–2.5 ng/mL) and blood hematocrit level (reference range, 39–51%). Blood samples were collected between 7–11 AM and all analyses were performed in the same laboratory.
Statistical analysis was performed using appropriate tests. Categorical variables were expressed as numbers (percentages), while numerical variables were presented as mean ± SD. Paired student-t-test was used to compare changes serum T, PSA and hematocrit levels before and after T therapy. A P value of <0.05 was considered statistically significant. All data were analyzed using SPSS® version 20 (IBM, Armonk, NY, USA).
Results
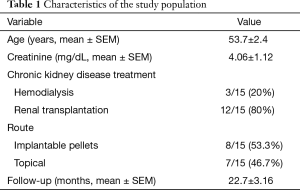
Fifteen patients were included in the study. Their mean age and serum creatinine levels ± SEM were 53.7±2.4 years and 4.06±1.12 mg/dL, respectively. T implants were utilized in 8/15 (53.3%) of patients, while topical T was used in the remaining 7/15 (46.7%) patients. All had bothersome symptoms including fatigue (15/15) and erectile dysfunction (ED) (14/15). The mean follow-up period ± SEM was 22.7±3.16 months (Table 1). Before T therapy, patients had a mean serum T of 207±20.1 ng/dL which significantly increased to 528±41.6 ng/dL after therapy. Likewise, a statistically significant increase in blood hematocrit level was detected after T therapy (35.8%±2.1% to 42.5%±1.4%) (Table 2). No major complications were reported. Three patients stopped T therapy; one because of exaggerated libido and two because of lack of symptom improvement in their ED. Prostate biopsy was performed in two patients because of an increase in PSA levels; however both had benign prostatic histopathology.
Full table

Full table
Discussion
We evaluated TRT in HD and renal transplant patients and found it a safe and successful treatment strategy capable of restoring normal T levels and improving symptoms. After a mean follow-up of 22.7 months, 80% of patients continued T therapy and had a mean T level of 528 ng/dL. Hypogonadism management in CKD patients has been practiced with caution due to the presumed adverse effects of T on hemodynamic and renal physiology. Such influences were principally derived from conducted animal studies. Blood pressure (BP) levels were found to be higher among male rats, whereas showed a drop to female levels after castration (9). This observation may be secondary to tubular renal sodium reabsorption and water retention that results from a T mediated stimulation of the intrarenal renin angiotensin system (RAS) (10). T can amplify the effects of angiotensin II through the activation of the Rho kinase signaling pathway, which intensifies renal vasoconstriction and promote chronic renal injury (11). RAS activation is also involved in kidney damage and fibrosis, independent of hypertension, an observation supported by a faster decline of renal function in men than women (12). Various inflammatory cytokines such as tumor necrosis factor a (TNFa), interleukin-1b (IL-1b), interleukin-6 (IL-6), and growth factors are implicated in renal inflammation and CKD progression (13) and appear to be intensified by T in vitro. Metcalfe et al. (14) revealed in castrated male rats, that exogenous administration of T stimulated TNF-a production and induced pro-apoptotic and pro-fibrotic pathways resulting in tubulointerstitial fibrosis and renal dysfunction. Despite all retrieved evidence from animal studies, such results may not be reproduced in human studies. For example several reports have demonstrated an inverse relationship between low endogenous T levels and hypertension (15,16), with BP reduction occurring after restoration of T levels to the normal range in hypogonadal men (17).
Albeit the above mentioned findings, a number of T-related favorable effects has been detected specifically in this subset of patients. Patients with CKD are at an increased risk of bone fractures in comparison to the general population (18). This risk results from several factors, including hyperparathyroidism, corticosteroid administration, osteoporosis and acidosis. An inverse relationship has been identified between bioavailable T levels and the degree of bone mineral density loss (19), and supported by the exaggerated bone resorption in elderly men with lower levels of serum T (20). Androgen can directly stimulate osteoblastic and osteoclastic receptors (21) and regulate various cytokines and growth factors such as insulin growth factors (IGFs) which are involved in bone formation and osteoclastogenesis (21). In a randomized placebo controlled clinical trial, Snyder et al. demonstrated a beneficial effect for T replacement on lumbar spine bone density most noticeable with lower pretreatment serum T concentrations (22).
Anemia is another consequence of CKD that is associated with increased morbidity and mortality. It has a multifactorial etiology; however, decreased erythropoietin (EPO) synthesis is perhaps the most adopted one. Androgens have been remotely used in the management of renal anemia in HD patients based on the direct simulating effect of T on erythropoiesis (23) and on its ability to enhance the sensitivity of erythroid progenitors to EPO (24). Moreover, T plays a major role in iron metabolism. It enhances the intestinal absorption of iron, suppresses the regulatory peptide hepcidin resulting in an increase in the bioavailability of iron (25) and finally, improves iron incorporation by erythrocytes. An increased hemoglobin concentration has been detected by Shaldon et al. (26) in 25 HD patients after intramuscular administration of T (250–500 mg weekly). A finding that has been confirmed by few other reports (27).
A reciprocal relationship between CKD and cardiovascular disease (CVD) exists with both disease entities being characterized by endothelial dysfunction, atherosclerosis, systemic inflammation and increased mortality (28). Hypogonadism has been associated with hypertension, systemic inflammation and increased mortality due to CVD in the general population (29). Cardiac disease is highly prevalent in HD patients affecting almost 80% at baseline making it the leading cause of death in this population (30). The significance of the relationship between androgens and CVD in renal patients was quantified by Yilmaz et al. (31). After investigating 239 male CKD patients, the authors reported a 22% decrease in the risk of cardiovascular events with each nanomole-per-liter increment in total T concentration (31). Karakitsos et al. (32) linked the hypogonadism seen in CKD patients to precise clinical measures of endothelial dysfunction, reporting an inverse correlation between T levels and intimal/medial thickness of the carotid artery and left ventricular mass index. Finally, T deficiency has been linked to a higher risk of all-cause mortality in male patients with CKD (33), with a more favorable overall prognosis seen in HD patients with higher T levels (34).
Although transplantation may revert many of the consequences of renal impairment, the state of hypogonadism can persist even after normalization of kidney function. Immunosuppressive medications are contributory as T production can be inhibited by glucocorticoids acting directly on gonadal steroid receptors or indirectly at the hypothalamic–pituitary level. Furthermore, cyclosporine-A and tacrolimus can also have a direct toxic effect on Leydig cells and on the hypothalamic-pituitary axis (35). Indeed, this study reveals that while the majority of patients (80%) had renal transplantation, their mean serum T level was low (207±20.1 ng/dL).
The small sample size and retrospective nature can be considered limitations to this study, however it adds to the previous small sized reports of a rather restraint clinical practice in this patient population.
Conclusions
Results of this study add to the already available evidence which supports the safety of TRT in patients with CKD. Statistically significant increments in serum T and hematocrit levels were reported and the majority of patients conveyed symptomatic improvement after treatment. Nonetheless, close monitoring is still advisable in CKD patients on TRT while awaiting larger prospective reports of safety and efficacy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board of the Cleveland Clinic of #10-1047.
References
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465-6. [Crossref] [PubMed]
- Spitzer M, Huang G, Basaria S, et al. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol 2013;9:414-24. [Crossref] [PubMed]
- Grossmann M, Hoermann R, Ng Tang Fui M, et al. Sex steroids levels in chronic kidney disease and kidney transplant recipients: associations with disease severity and prediction of mortality. Clin Endocrinol (Oxf) 2015;82:767-75. [Crossref] [PubMed]
- Albaaj F, Sivalingham M, Haynes P, et al. Prevalence of hypogonadism in male patients with renal failure. Postgrad Med J 2006;82:693-6. [Crossref] [PubMed]
- Brockenbrough AT, Dittrich MO, Page ST, et al. Transdermal androgen therapy to augment EPO in the treatment of anemia of chronic renal disease. Am J Kidney Dis 2006;47:251-62. [Crossref] [PubMed]
- Singh AB, Norris K, Modi N, et al. Pharmacokinetics of a transdermal testosterone system in men with end stage renal disease receiving maintenance hemodialysis and healthy hypogonadal men. J Clin Endocrinol Metab 2001;86:2437-45. [PubMed]
- Cangüven O, Aykose G, Albayrak S, et al. Efficacy of testosterone gel in the treatment of erectile dysfunction in hypogonadal hemodialysis patients: a pilot study. Int J Impot Res 2010;22:140-5. [Crossref] [PubMed]
- Lawrence IG, Price DE, Howlett TA, et al. Correcting impotence in the male dialysis patient: experience with testosterone replacement and vacuum tumescence therapy. Am J Kidney Dis 1998;31:313-9. [Crossref] [PubMed]
- Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 1998;31:435-9. [Crossref] [PubMed]
- Quan A, Chakravarty S, Chen JK, et al. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol 2004;287:F452-9. [Crossref] [PubMed]
- Song J, Eyster KM, Kost CK Jr, et al. Involvement of protein kinase C-CPI-17 in androgen modulation of angiotensin II-renal vasoconstriction. Cardiovasc Res 2010;85:614-21. [Crossref] [PubMed]
- Ji H, Menini S, Mok K, et al. Gonadal steroid regulation of renal injury in renal wrap hypertension. Am J Physiol Renal Physiol 2005;288:F513-20. [Crossref] [PubMed]
- D'Agostino P, Milano S, Barbera C, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y Acad Sci 1999;876:426-9. [Crossref] [PubMed]
- Metcalfe PD, Leslie JA, Campbell MT, et al. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab 2008;294:E435-43. [Crossref] [PubMed]
- Svartberg J. Epidemiology: testosterone and the metabolic syndrome. Int J Impot Res 2007;19:124-8. [Crossref] [PubMed]
- Svartberg J, von Mühlen D, Schirmer H, et al. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol 2004;150:65-71. [Crossref] [PubMed]
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab 2007;92:3844-53. [Crossref] [PubMed]
- Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000;58:396-9. [Crossref] [PubMed]
- Ishani A, Paudel M, Taylor BC, et al. Renal function and rate of hip bone loss in older men: the Osteoporotic Fractures in Men Study. Osteoporos Int 2008;19:1549-56. [Crossref] [PubMed]
- Falahati-Nini A, Riggs BL, Atkinson EJ, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 2000;106:1553-60. [Crossref] [PubMed]
- Vanderschueren D, Vandenput L, Boonen S, et al. Androgens and bone. Endocr Rev 2004;25:389-425. [Crossref] [PubMed]
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999;84:1966-72. [PubMed]
- Parker JP, Beirne GJ, Desai JN, et al. Androgen-induced increase in red-cell 2,3-diphosphoglycerate. N Engl J Med 1972;287:381-3. [Crossref] [PubMed]
- Ballal SH, Domoto DT, Polack DC, et al. Androgens potentiate the effects of erythropoietin in the treatment of anemia of end-stage renal disease. Am J Kidney Dis 1991;17:29-33. [Crossref] [PubMed]
- Bachman E, Feng R, Travison T, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab 2010;95:4743-7. [Crossref] [PubMed]
- Shaldon S, Koch KM, Oppermann F, et al. Testosterone therapy for anaemia in maintenance dialysis. Br Med J 1971;3:212-5. [Crossref] [PubMed]
- Schustack A, Meshiaj D, Waiss Z, et al. Intramuscular iron replenishment and replacement combined with testosterone enanthate in maintenance hemodialysis anemia: a follow-up of up to 8 years on 16 patients. Clin Nephrol 1985;23:303-6. [PubMed]
- Zoccali C. Endothelial dysfunction and the kidney: emerging risk factors for renal insufficiency and cardiovascular outcomes in essential hypertension. J Am Soc Nephrol 2006;17:S61-3. [Crossref] [PubMed]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 2008;93:68-75. [Crossref] [PubMed]
- Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int 2004;65:2380-9. [Crossref] [PubMed]
- Yilmaz MI, Sonmez A, Qureshi AR, et al. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol 2011;6:1617-25. [Crossref] [PubMed]
- Karakitsos D, Patrianakos AP, De Groot E, et al. Androgen deficiency and endothelial dysfunction in men with end-stage kidney disease receiving maintenance hemodialysis. Am J Nephrol 2006;26:536-43. [Crossref] [PubMed]
- Haring R, Nauck M, Völzke H, et al. Low serum testosterone is associated with increased mortality in men with stage 3 or greater nephropathy. Am J Nephrol 2011;33:209-17. [Crossref] [PubMed]
- Majdan M, Kotarski J, Ksiaźek A, et al. Relationship between some prognostic markers of HD patients and serum erythropoietin, insulin-like growth factor-1, leptin, parathormone and testosterone. Int Urol Nephrol 1999;31:563-9. [Crossref] [PubMed]
- Schmidt A, Luger A, Hörl WH. Sexual hormone abnormalities in male patients with renal failure. Nephrol Dial Transplant 2002;17:368-71. [Crossref] [PubMed]


