Retrograde ejaculation, painful ejaculation and hematospermia
Introduction
Although there has been an increased interest in premature ejaculation since the introduction of the first oral compound developed specially for the treatment of this problem in 2006 (1), our understanding regarding the disorders of retrograde ejaculation, painful ejaculation and hematospermia remain limited.
In this article, we will summarize the possible etiologic factors related to these ejaculatory problems and their management will be reviewed in light of the current literature.
Retrograde ejaculation
Ejaculation is an essential step in normal human reproduction and its failure leads to infertility. Many ejaculatory disorders can have both psychological as well as organic causes; however, retrograde ejaculation is unique in that as it is almost exclusively organic in origin. Despite being a common type of ejaculatory dysfunction, it is responsible for only 0.3–2% of infertility (2). The combination of dry orgasm and issue with fertility make the condition distressing to both patient and their partner especially when trying to conceive (3).
The process of ejaculation requires complex co-ordination and interplay between the epidiymides, vasa deferentia, prostate, seminal vesicles, bladder neck and bulbourethral glands (4). Upon ejaculation, sperm are rapidly conveyed along the vas deferens and into the urethra via the ejaculatory ducts. From there, the semen progresses in an antegrade fashion in part maintained by coaptation of the bladder neck and rhythmic contraction of the periurethral muscles co-ordinated by a centrally mediated reflex. Closure of the bladder neck and seminal emission are initiated via the sympathetic nervous system from the lumbar sympathetic ganglia and subsequently hypogastric nerve. Prostatic and seminal vesicle secretion as well as contraction of the bulbocavernosal, ischiocavernosal and pelvic floor are initiated by the S2-4 parasympathetic nervous system via the pelvic nerve.
Any factor, which disrupts this reflex and inhibits the bladder neck (internal vesical sphincter) contraction, may lead to retrograde passage of semen into the bladder. These can be broadly categorised as pharmacological, neurogenic or anatomic causes of retrograde ejaculation (Table 1).

Full table
Men with retrograde ejaculation have little to suggest a diagnosis in terms of symptoms beyond that of reduced ejaculation or dry orgasm. Post orgasm, many men will describe the passage of cloudy urine. This can be attributed to the mixing of semen in the bladder with urine. A number of men will present with fertility issues for the obvious reasons (5). A thorough history should focus on the timing and symptoms as well as identifying the underlying cause.
The lower reference limit for semen volume is 1.5 mL [5th centile, 95% confidence interval (CI) 1.4–1.7] as defined by the World Health Organisation (6). Men with values below this are considered to have hypospermia whilst those with complete absence of ejaculate are defined as aspermic. These terms should not be confused with oligozoospermia and azoospermia that indicate reduced sperm counts but can have a normal semen volume. Hypospermia, as defined by the National Institute of Health, is a condition when a semen volume lower than 2 mL is recorded on at least two semen analyses (7). Hypospermia or aspermia should highlight to the clinician the possibility of retrograde ejaculation. The presence of either though cannot differentiate between a disorder of emission and true retrograde ejaculation.
Consequently, Vroege et al. suggested that the analysis and confirmation of sperm in a post orgasmic urine sample could help differentiate between these two separate disorders (8). Fructose can also be found in the urine analysis after orgasm in patients with retrograde ejaculation (9). McMahon et al. recommended that the post-orgasmic urine should be centrifuged and the visualization of 10–15 sperm per high-power field would confirm the diagnosis of retrograde ejaculation (10) whereas Fedder et al. defined retrograde ejaculation as more than one million spermatozoa found in a post-ejaculatory urine sample (5).
Medical and surgical strategies exist for the treatment of retrograde ejaculation. In recent years the reliance of medical treatment as first line management has become common practice. Sympathomimetics stimulate the release of noradrenaline as well as activating alpha- and beta-adrenergic receptors, resulting in closure of the internal urethral sphincter, restoring antegrade flow of semen. The most common sympathomimetics are synephrine, pseudoephedrine hydrochloride, ephedrine, phenylpropanolamine and midrodrine. Unfortunately as time progresses their effect diminishes (11). Many of the studies published about the efficacy of sympathomimetics in the treatment of retrograde ejaculation suffer from small sample size with some represented by case reports.
A double blind controlled study randomised patients to one of four alpha-adrenergic agents (dextroamphetamine, ephedrine, phenylpropanolamine and pseudoephedrine) with or without histamine. The patients suffered from failure of ejaculation following retroperitoneal lymphadenectomy. They found that 4 days of treatment prior to ejaculation was most effective and that all the adrenergic agonists restored antegrade ejaculation (12).
In a systematic review, the efficacy of this group of medications was found to be 28% (2). The side effects of sympathomimetics include dryness of mucous membranes and hypertension.
The use of antimuscarinics has been described, including brompheniramine maleate and imipramine, as well as in combination with sympathomimetics. The calculated efficacy of antimuscarinics or antimuscariics in combination with sympathomimetics are 22% vs. 39% respectively (2). Combination therapy appears to be more effective although statistical analysis is not yet possible due to the small sample sizes.
Bladder neck reconstruction has also been suggested for the treatment of retrograde ejaculation. Abrahams described the use of a V-Y plasty of the bladder neck in two patients who subsequently regained antegrade ejaculatory function and one went on to father a child (13). Similarly, Middleton and Urry used the Young-Dees type of bladder neck reconstruction and were able to restore normal antegrade ejaculation in 4 out of 5 patients (14). Other surgical or interventional techniques have included injecting collagen into the bladder neck and the use of surgical sperm retrieval to achieve pregnancy (15).
Infertility has been the major concern of patients with retrograde ejaculation. Beyond the use of standard sperm retrieval techniques such as TESE and PESA, three different methods of sperm retrieval have been identified for the management of infertility in the patient suffering from retrograde ejaculation. These include; centrifugation and resuspension of post ejaculatory urine specimens, the Hotchkiss (or modified Hotchkiss) technique and ejaculation on a full bladder:
- Centrifugation and resuspension: in order to improve the ambient conditions for the sperm, the patient is asked to either increase their fluid intake or to take sodium bicarbonate to dilute or alkalise the urine respectively. Afterwards, a post orgasmic urine sample is collected by either introducing a catheter or spontaneous voiding. This sample is then centrifuged and suspended in a medium. The types of suspension fluids employed are heterogeneous and can include bovine serum albumin, human serum albumin, Earle’s/Hank’s, phosphate buffered medium and the patients urine to name but a few. The resultant modified sperm mixture can then be used in assisted reproductive techniques. A systematic review of the literature in couples with the male partner suffering from retrograde ejaculation found a 15% pregnancy rate per cycle (0–100%) based on 15 articles (2);
- Hotchkiss method: the Hotchkiss and modified Hotchkiss method involves emptying the bladder prior to ejaculation using a catheter and then washing out and instilling a small quantity of Lactated Ringers to improve the ambient conditions of the bladder. The patient then ejaculates and semen is retrieved by catheterisation or voiding (16). Modified Hotchkiss methods involve a variance in the instillation medium. Pregnancy rates per cycle were 24% per cycle (0–100%) based on eight papers (2);
- Ejaculation on a full bladder: few papers have described results from this technique (17,18). The patient is encouraged to ejaculate on a full bladder and semen is suspended in Baker’s buffer. The pregnancy rate in the two studies which included only five patients in total was 60% (2).
Painful ejaculation
Painful ejaculation (also known as dysejaculation, odynorgasmia, post orgasmic pain, dysorgasmia or orgasmalgia) is a common but poorly understood clinical phenomenon, which is associated with sexual dysfunction. Several studies demonstrated its prevalence in between 1–10% in the general population (19-21); however, it may increase to 30–75% among men who suffer from chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) (22-26). It should be noted that the design of the majority of these papers is not scientifically sound and the condition is probably underreported due to the lack of an evidence-based definition and well-defined prognostic criteria.
The severity of painful ejaculations may vary from a minor discomfort to excruciating pain (27-31). It may occur anywhere in the pudendal territory, including penis, scrotum and perineal/perianal region (27-29,31-36). The pain typically initiates immediately before or during ejaculation and commonly lasts between 2 to 24 hours (32,33). This problem may reduce the individual’s self-esteem and sexual desire, which could well result in a decrease quality of life (37).
Many medical conditions can result in painful ejaculations but it can also be an idiopathic problem. Initial reports demonstrated possible associations of painful ejaculation with calculi in the seminal vesicles, sexual neurasthenia, sexually transmitted diseases (38-40) antidepressants (28,29,41,42), inflammation of prostate (25,43), prostate cancer (44,45), benign prostatic hyperplasia (23,46-49), prostate surgery (50,51), pelvic radiation (52) and herniorrhaphy (27,30,34,53) amongst others.
Psychological issues may also be the cause of painful ejaculations, especially if the patient does not experience this problem during masturbation (54-56). Several case reports have suggested that mercury toxicity or ciguatera toxin fish poisoning may also result in painful ejaculations (57-59).
Common to all sexual problems, the assessment of a patient with painful ejaculation must start with a rigorous medical and sexual history. Such a history should relate to the following as a minimum-relationship issues, psychological problems, sexually transmitted diseases, drug intake, urinary symptoms, prostatic diseases (e.g., prostatitis, CP/CPPS), familial prostate cancer, previous surgical procedures (e.g., groin herniorrhaphy, radical prostatectomy) and radiotherapy must be recorded.
Several questionnaires have been developed and should be ideally be administered—such as Beck depression and anxiety scales, International Prostatic Symptom Score (IPSS), National Institute of Health-Chronic Prostatitis Symptom Index (NIH-CPSI). These will assist the physician in ascertaining the underlying patient condition at an early stage. The type and location of the pain (visceral neuropathic or somatic) must be recorded (60) along with those circumstances which aggravate and relieve pain.
A focused physical examination may disclose scars from previous surgeries or radiotherapy in the groin area whereas pathognomonic dermal lesions and purulent urethral discharge may be suggestive of sexually transmitted diseases. Palpation of a swollen and painful prostate during digital rectal examination (DRE) is a diagnostic finding for acute prostatitis whereas a nodule can be felt in the presence of a prostate cancer. A neurological and musculoskeletal examination may detect pudendal neuropathy which is caused by pudendal nerve entrapment, pudendal canal syndrome or pudendal neuralgia (61).
Laboratory tests should be focused on symptoms. Direct microscopic examination of the urethral discharge and urethral culture may be beneficial in assigning the type of the bacteria, which causes urethritis. Similarly, a 4-cup test may confirm the location of urinary infection and confirm the diagnosis of prostatitis.
Serum prostate specific antigen levels may also suggest pathology within the prostate (i.e., prostatitis and/or prostate cancer). Abdominal computerized tomography scans are rarely required. Magnetic resonance imaging studies may be helpful when investigating the cause of pudendal neuropathy. However, no obvious aetiology is found in a significant number of patients with the complaint of painful ejaculations, despite extensive investigation (62).
Treatment of painful ejaculation must be tailored according to the underlying cause, if detected. Psychotherapy or relationship counselling, withdrawal of suspected agents (drugs, toxins, or radiation) (28,29,41,42) or the prescription of appropriate medical treatment (antibiotics, α-blockers, anti-inflammatory agents) may ameliorate painful ejaculations (63). If medical treatments fail, surgical operations such as transurethral resection of the prostate, transurethral resection of the ejaculatory duct and neurolysis of the pudendal nerve may be necessary (64-66). Behavioural therapies, myorelaxant, antidepressant pelvic floor exercises, anticonvulsant drugs and/or opioids may be administered if no underlying cause can be identified (67,68).
Hemospermia
The presence of blood in the semen can cause the patient significant anxiety although it is considered by many professionals to be a self-limiting and usually benign condition (69,70). Comments on the symptom have been passed by notaries including Hippocrates, Galen, Pare, Morgagni, and Fournier (69). The few articles that exist in the literature are limited mainly to case reports and cohort series. Consequently, there is little to recommend a definitive diagnostic or management strategy.
The definition of hemospermia is the presence of blood in the ejaculate. Further classification is not mentioned in the literature and there is no distinction between visible and non-visible hemospermia.
The exact incidence and prevalence of hemospermia is difficult to elucidate due to a number of factors including its covert presentation, usually self-limiting nature and patient embarrassment. The symptom represents approximately 1–1.5% of all urological referrals and occurs in all age groups with a mean age of 37 (69,71). It is usually a self-limiting condition with an average duration of 1–24 months (69,70).
In a prostate cancer screening study of 26,126 men, 50 years and older or older than 40 with a history of prostate cancer or of black race, hemospermia was found in 0.5% on entry to the trial (72).
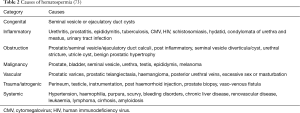
A number of causes of hematospermia have been identified but can be broadly classified into the following sub-categories; idiopathic, congenital malformations, inflammatory conditions, obstruction, malignancies, vascular abnormalities, iatrogenic/trauma and systemic causes (Table 2).
The risk of any malignancy in patients presenting with hematospermia is on average 3.5% (range, 0–13.1%) (74). In an observational study of 300 consecutive patients over a 30-month period, 81% had no cause of their hematospermia identified. In those patients for whom a cause was identified, the diagnosis varied dependent upon the age of presentation. When the patients were divided into those under and over 40 years of age, urinary tract infections were more common among younger patients vs. older patients (15% vs. 10.3%). In the older group (>40 years old), stones (2.2% vs. 1.4%) and malignancy (6.2% vs. 1.4%) were more common when compared with the younger cohort (71). In the over 40 group, 13 patients had prostate cancer and 1 had a low-grade urethral carcinoma. In the under 40 group, 1 patient had testicular cancer (71).
The investigation of hematospermia should begin with a thorough symptom specific and systemic clinical history—accompanied by examination of the patient. The first step is to establish if the patient has true hematospermia. Pseudo-hematospermia can be as a consequence of haematuria or even suction of a partner’s blood into the urethra during copulation. This can be excluded by performing a condom test and the semen visually examined. Melanospermia that is a consequence of malignant melanoma involving the genitourinary tract has also been described in a two case reports (75,76). Chromatography of the semen sample can be used to distinguish the two by identifying the presence of melanin. The volume, colour, duration and frequency should be noted along with the age and if it is recurrent or isolated.
Associated features should also be elicited including lower urinary tract symptoms (LUTS), concomitant haematuria and testicular/penile pain. A sexual history should be taken to identify those whose hematospermia may be as a consequence of a sexually transmitted disease. Recent foreign travel to areas affected by schistosomiasis or tuberculosis should also be considered. The possibility of co-existing systemic disease such as hypertension, liver disease and coagulopathies should be investigated along with systemic features of malignancy such as weight loss, loss of appetite or bony pain.
Examination of the patient should include measurement of the blood pressure, as there have been a number of case reports suggesting an association between uncontrolled hypertension and hematospermia (77,78). The abdomen should be examined for masses and organomegaly. A thorough groin, perineal, and genital examination should be performed including the urethral meatus.
DRE should also be performed, and the meatus re-examined after DRE for the presence of bloody discharge (79). Detection of a palpable nodule in the prostate is of importance as an association between hematospermia and prostate cancer has been postulated although not completely proven. A community-based prostate screening study was undertaken of 26,126 men over 50 (or older than 40 with a family history of prostate cancer of black American heritage) who underwent PSA testing and DRE at 6 monthly or yearly intervals. At each visit they were asked about the presence of hemospermia, haematuria and LUTS. Patients with a raised PSA underwent transrectal ultrasound (TRUS) guided prostate biopsy. In the overall screening population, a significantly higher number of patient with hemospermia were found to have prostate cancer compared with those that did not have hemospermia (13.7% vs. 6.5%) (72). The same held true when men with hemospermia and prostate cancer were matched with those diagnosed with prostate cancer and no hemospermia (P=0.035) (72).
On multivariate logistic regression modelling, after adjusting for age, PSA and DRE, hemospermia was predictive of prostate cancer although narrowly missed significance (72). The fact that this study though was part of a prostate-screening group suggests that the results should be interpreted cautiously due to the risk of bias.
Most authors that propose an investigative baseline agree on the initial diagnostic tests, however there is no consensus statement in this regards (73,74,80,81).
A urinalysis should be taken along with sending the urine for culture and sensitivities as well as microscopy. If tuberculosis or schistosomiasis is suspected, the semen or prostatic secretions should be sent for analysis. A full sexually transmitted disease screen including first void urine as well as serum and genitourinary samples should be taken and tested for chlamydia, ureaplasma and herpes simplex. Using this strategy, it may be possible to find an infectious agent among patients who would have been labelled as idiopathic hematospermia (82).
A serum PSA should be taken in men over the age of 40 who have been appropriately counselled as previously mentioned (72). Blood work including a full blood count, liver function tests, and a clotting screen should be taken to identify systemic diseases as per Table 2. The question of whether further investigation is warranted depends on clinician judgement, patient age and an assessment of risk factors (73).
Prior to the widespread accessibility of TRUS, MRI and CT, the standard imaging was a plain X-ray KUB and an excretory intravenous urogram. However, these modern imaging modalities are more sensitive in diagnosis of malignant disease (83). TRUS is now widely accepted as the first imaging modality in men presenting with risk features. It is relatively cheap, accessible and enables real-time cross sectional imaging. TRUS is able to identify stones in the seminal vesicles, prostate and ejaculatory ducts, and soft tissue masses such as polyps and tumour. Further biopsy of any soft tissue abnormalities can also be taken at the time.
There are a number of papers that recommend its use and have suggested that it can demonstrate an abnormality in 82–95% of men with hematospermia (70,84-88).
In a recent study of 115 patients presenting with hematospermia, all patients had a benign finding on TRUS (85.2% had calcification, 7% hypoechoic area, 30.4% had a seminal vesical abnormality) (89). Earlier studies by Yagci et al. and Zhao et al. had similar findings with abnormalities found in 94.5% and 94.8% respectively in men referred for hematospermia (85).
MRI is being increasingly used as a definitive means to investigate hematospermia. The multiplanar ability of MRI to accurately represent structural changes in the prostate, seminal vesicles, ampulla of vas deferens, and ejaculatory duct has enabled the modality to be particularly useful in determining the organ of origin of midline or paramedian prostatic cysts and in determining optimal surgical management (90-94). The addition of an endorectal coil can improve the diagnostic accuracy for identifying the site and case of haemorrhage (90, 91,94).
The use of cystoscopy has been included in the majority of suggested investigation protocols in patients with high-risk features. It can provide invaluable information as it allows direct visualisation of the main structures in the urinary tract that can be attributed to causes of hematospermia such as polyps, urethritis, prostatic cysts, foreign bodies, calcifications and vascular abnormalities (74).
With the advancement of optics the ability to create ureteroscopes of diameters small enough to allow insertion into the ejaculatory duct and seminal vesicles has been made possible. The first attempt to use a ureteroscope to directly visualise the seminal vesical was performed in 1997 to investigate a fistula (95).
Subsequently a number of articles have been submitted examining the diagnostic and therapeutic advantages of this approach (96-101). In a prospective study of 106 patients with prolonged hematospermia patients underwent both TRUS and seminal vesiculoscopy. With both modalities combined, diagnoses were made in 87.7% of patients. When compared, head-to-head, the diagnostic yield for TRUS vs. seminal vesiculoscopy was 45.3% vs. 74.5% respectively (P<0.001) (102).
Conclusions
All three of the conditions that have been explored in this article require a keen clinical acumen and willingness to engage in thinking outside of the standard established treatment paradigm. The development of novel investigational techniques and treatments has led to progress in the management of these conditions symptoms; however, the literature almost uniformly is limited to small series and rare randomised trials. Further investigation and randomised controlled trials are needed for progress in these often challenging cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pryor JL, Althof SE, Steidle C, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet 2006;368:929-37. [Crossref] [PubMed]
- Jefferys A, Siassakos D, Wardle P. The management of retrograde ejaculation: a systematic review and update. Fertil Steril 2012;97:306-12. [Crossref] [PubMed]
- Rowland D, McMahon CG, Abdo C, et al. Disorders of orgasm and ejaculation in men. J Sex Med 2010;7:1668-86. [Crossref] [PubMed]
- Giuliano F, Clement P. Neuroanatomy and physiology of ejaculation. Annu Rev Sex Res 2005;16:190-216. [PubMed]
- Fedder J, Kaspersen MD, Brandslund I, et al. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology 2013;1:602-6. [Crossref] [PubMed]
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45. [Crossref] [PubMed]
- Robin G, Marcelli F, Mitchell V, et al. Why and how to assess hypospermia? Gynecol Obstet Fertil 2008;36:1035-42. [Crossref] [PubMed]
- Vroege JA, Gijs L, Hengeveld MW. Classification of sexual dysfunctions: towards DSM-V and ICD-11. Compr Psychiatry 1998;39:333-7. [Crossref] [PubMed]
- Cruz N, Porst H. Ejaculatory and orgasmic disorders other than premature ejaculation. In: Porst H, Reisman Y, eds. The ESSM Syllabus of Sexual Medicine. European Society for Sexual Medicine (ESSM) and Medix Publishers BV, 2012:737-90.
- McMahon CG. Disorders of Male Orgasm and Ejaculation. In: Wein AJ, Kavoussi LR, Partin AW, et al. eds. Campbell-Walsh Urology. Philadelphia: Elsevier, 2016;692-708.
- Gilja I, Parazajder J, Radej M, et al. Retrograde ejaculation and loss of emission: possibilities of conservative treatment. Eur Urol 1994;25:226-8. [PubMed]
- Proctor KG, Howards SS. The effect of sympathomimetic drugs on post-lymphadenectomy aspermia. J Urol 1983;129:837-8. [PubMed]
- Abrahams JI, Solish GI, Boorjian P, et al. The surgical correction of retrograde ejaculation. J Urol 1975;114:888-90. [PubMed]
- Middleton RG, Urry RL. The Young-Dees operation for the correction of retrograde ejaculation. J Urol 1986;136:1208-9. [PubMed]
- Reynolds JC, McCall A, Kim ED, et al. Bladder neck collagen injection restores antegrade ejaculation after bladder neck surgery. J Urol. 1998;159:1303. [Crossref] [PubMed]
- Hotchkiss RS, Pinto AB, Kleegman S. Artificial insemination with semen recovered from the bladder. Fertil Steril 1954;6:37-42. [Crossref] [PubMed]
- Templeton A, Mortimer D. Successful circumvention of retrograde ejaculation in an infertile diabetic man. Case report. Br J Obstet Gynaecol 1982;89:1064-5. [Crossref] [PubMed]
- Crich JP, Jequier AM. Infertility in men with retrograde ejaculation: the action of urine on sperm motility, and a simple method for achieving antegrade ejaculation. Fertil Steril 1978;30:572-6. [Crossref] [PubMed]
- Líndal E, Stefànsson JG. The lifetime prevalence of psychosexual dysfunction among 55 to 57-year-olds in Iceland. Soc Psychiatry Psychiatr Epidemiol 1993;28:91-5. [Crossref] [PubMed]
- Blanker MH, Bosch JL, Groeneveld FP, et al. Erectile and ejaculatory dysfunction in a community-based sample of men 50 to 78 years old: prevalence, concern, and relation to sexual activity. Urology 2001;57:763-8. [Crossref] [PubMed]
- Roberts RO, Jacobson DJ, Girman CJ, et al. Prevalence of prostatitis-like symptoms in a community based cohort of older men. J Urol 2002;168:2467-71. [Crossref] [PubMed]
- Sönmez NC, Kiremit MC, Güney S, et al. Sexual dysfunction in type III chronic prostatitis (CP) and chronic pelvic pain syndrome (CPPS) observed in Turkish patients. Int Urol Nephrol 2011;43:309-14. [Crossref] [PubMed]
- Nickel JC, Elhilali M, Vallancien G, et al. Benign prostatic hyperplasia (BPH) and prostatitis: prevalence of painful ejaculation in men with clinical BPH. BJU Int 2005;95:571-4. [Crossref] [PubMed]
- Mo MQ, Long LL, Xie WL, et al. Sexual dysfunctions and psychological disorders associated with type IIIa chronic prostatitis: a clinical survey in China. Int Urol Nephrol 2014;46:2255-61. [Crossref] [PubMed]
- Wagenlehner FM, van Till JW, Magri V, et al. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur Urol 2013;63:953-9. [Crossref] [PubMed]
- Shoskes DA, Landis JR, Wang Y, et al. Impact of post-ejaculatory pain in men with category III chronic prostatitis/chronic pelvic pain syndrome. J Urol 2004;172:542-7. [Crossref] [PubMed]
- Butler JD, Hershman MJ, Leach A. Painful ejaculation after inguinal hernia repair. J R Soc Med 1998;91:432-3. [PubMed]
- Michael A. Venlafaxine-induced painful ejaculation. Br J Psychiatry 2000;177:282. [Crossref] [PubMed]
- Demyttenaere K, Huygens R. Painful ejaculation and urinary hesitancy in association with antidepressant therapy: relief with tamsulosin. Eur Neuropsychopharmacol 2002;12:337-41. [Crossref] [PubMed]
- Aasvang EK, Møhl B, Kehlet H. Ejaculatory pain: a specific postherniotomy pain syndrome? Anesthesiology 2007;107:298-304. [Crossref] [PubMed]
- Calabrò RS, Marra A, Quattrini F, et al. Central neuropathic pain: an unusual case of painful ejaculation responding to topiramate. J Sex Med 2012;9:3274-8. [Crossref] [PubMed]
- Barnas JL, Pierpaoli S, Ladd P, et al. The prevalence and nature of orgasmic dysfunction after radical prostatectomy. BJU Int 2004;94:603-5. [Crossref] [PubMed]
- Barnas J, Parker M, Guhring P, et al. The utility of tamsulosin in the management of orgasm-associated pain: a pilot analysis. Eur Urol 2005;47:361-5; discussion 365. [Crossref] [PubMed]
- Aasvang EK, Møhl B, Bay-Nielsen M, et al. Pain related sexual dysfunction after inguinal herniorrhaphy. Pain 2006;122:258-63. [Crossref] [PubMed]
- Ilie CP, Mischianu DL, Pemberton RJ. Painful ejaculation. BJU Int 2007;99:1335-9. [Crossref] [PubMed]
- Davis SN, Maykut CA, Binik YM, et al. Tenderness as measured by pressure pain thresholds extends beyond the pelvis in chronic pelvic pain syndrome in men. J Sex Med 2011;8:232-9. [Crossref] [PubMed]
- Vallancien G, Emberton M, Harving N, et al. Sexual dysfunction in 1,274 European men suffering from lower urinary tract symptoms. J Urol 2003;169:2257-61. [Crossref] [PubMed]
- Edwards A. Chronic disease of the colliculus seminalis. Br Med J 1909;2:1672-3. [Crossref] [PubMed]
- Grosse AB. Remarks on Impotentia Cocundi and Sexual Neurasthenia and Their Treatment. Cal State J Med 1911;9:25-7. [PubMed]
- Irwin WK. Pain in genito-urinary affections: Its variations and their Interpretation. Br Med J 1922;2:457-8. [Crossref] [PubMed]
- Kulik FA, Wilbur R. Case report of painful ejaculation as a side effect of amoxapine. Am J Psychiatry 1982;139:234-5. [Crossref] [PubMed]
- Aizenberg D, Zemishlany Z, Hermesh H, et al. Painful ejaculation associated with antidepressants in four patients. J Clin Psychiatry 1991;52:461-3. [PubMed]
- Tran CN, Shoskes DA. Sexual dysfunction in chronic prostatitis/chronic pelvic pain syndrome. World J Urol 2013;31:741-6. [Crossref] [PubMed]
- Walz J, Perrotte P, Gallina A, et al. Ejaculatory disorders may affect screening for prostate cancer. J Urol 2007;178:232-7; discussion 237-8. [Crossref] [PubMed]
- Kleinberg L, Wallner K, Roy J, et al. Treatment-related symptoms during the first year following transperineal 125I prostate implantation. Int J Radiat Oncol Biol Phys 1994;28:985-90. [Crossref] [PubMed]
- Li MK, Garcia L, Patron N, et al. An Asian multinational prospective observational registry of patients with benign prostatic hyperplasia, with a focus on comorbidities, lower urinary tract symptoms and sexual function. BJU Int 2008;101:197-202. [PubMed]
- Rosen RC, Fitzpatrick JM. ALF-LIFE Study Group. Ejaculatory dysfunction in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. BJU Int 2009;104:974-83. [Crossref] [PubMed]
- Gacci M, Eardley I, Giuliano F, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 2011;60:809-25. [Crossref] [PubMed]
- Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol 2003;44:637-49. [Crossref] [PubMed]
- Koeman M, van Driel MF, Schultz WC, et al. Orgasm after radical prostatectomy. Br J Urol 1996;77:861-4. [Crossref] [PubMed]
- Matsushita K, Tal R, Mulhall JP. The evolution of orgasmic pain (dysorgasmia) following radical prostatectomy. J Sex Med 2012;9:1454-8. [Crossref] [PubMed]
- Merrick GS, Wallner K, Butler WM, et al. Short-term sexual function after prostate brachytherapy. Int J Cancer 2001;96:313-9. [Crossref] [PubMed]
- Verhagen T, Loos MJ, Scheltinga MR, et al. Surgery for chronic inguinodynia following routine herniorrhaphy: beneficial effects on dysejaculation. Hernia 2016;20:63-8. [Crossref] [PubMed]
- Gruver GG. Functional male dyspareunia: a case study. Am J Psychother 1977;31:450-55. [PubMed]
- Kaplan HS. Post-ejaculatory pain syndrome. J Sex Marital Ther 1993;19:91-103. [Crossref] [PubMed]
- Schover LR. Psychological factors in men with genital pain. Cleve Clin J Med 1990;57:697-700. [Crossref] [PubMed]
- Lange WR, Lipkin KM, Yang GC. Can ciguatera be a sexually transmitted disease? J Toxicol Clin Toxicol 1989;27:193-7. [Crossref] [PubMed]
- Bluhm RE, Breyer JA, Bobbitt RG, et al. Elemental mercury vapour toxicity, treatment, and prognosis after acute, intensive exposure in chloralkali plant workers. Part II: Hyperchloraemia and genitourinary symptoms. Hum Exp Toxicol 1992;11:211-5. [Crossref] [PubMed]
- Senthilkumaran S, Balamurgan N, Suresh P, et al. Painful ejaculation. Something fishy. Saudi Med J 2010;31:451-2. [PubMed]
- Loos MJ, Roumen RM, Scheltinga MR. Classifying post-herniorrhaphy pain syndromes following elective inguinal hernia repair. World J Surg 2007;31:1760-5; discussion 1766-7.
- Durante JA, Macintyre IG. Pudendal nerve entrapment in an Ironman athlete: a case report. J Can Chiropr Assoc 2010;54:276-81. [PubMed]
- Luzzi GA, Law LA. The male sexual pain syndromes. Int J STD AIDS 2006;17:720-6. [Crossref] [PubMed]
- Anothaisintawee T, Attia J, Nickel JC, et al. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA 2011;305:78-86. [Crossref] [PubMed]
- Tuhkanen K, Heino A, Aaltoma S, et al. Sexual function of LUTS patients before and after neodymium laser prostatectomy and transurethral resection of prostate. A prospective, randomized trial. Urol Int 2004;73:137-42. [Crossref] [PubMed]
- Johnson CW, Bingham JB, Goluboff ET, et al. Transurethral resection of the ejaculatory ducts for treating ejaculatory symptoms. BJU Int 2005;95:117-9. [Crossref] [PubMed]
- Heise CP, Starling JR. Mesh inguinodynia: a new clinical syndrome after inguinal herniorrhaphy? J Am Coll Surg 1998;187:514-8. [Crossref] [PubMed]
- Jordi P, Maria-José A, Luis-Alfonso M, et al. Management of ejaculation pain with topiramate: a case report. Clin J Pain 2004;20:368-9. [Crossref] [PubMed]
- Cornel EB, van Haarst EP, Schaarsberg RW, et al. The effect of biofeedback physical therapy in men with Chronic Pelvic Pain Syndrome Type III. Eur Urol 2005;47:607-11. [Crossref] [PubMed]
- Mulhall JP, Albertsen PC. Hemospermia: diagnosis and management. Urology 1995;46:463-7. [Crossref] [PubMed]
- Amano T, Kunimi K, Ohkawa M. Transrectal ultrasonography of the prostate and seminal vesicles with hemospermia. Urol Int 1994;53:139-42. [Crossref] [PubMed]
- Ng YH, Seeley JP, Smith G. Haematospermia as a presenting symptom: outcomes of investigation in 300 men. Surgeon 2013;11:35-8. [Crossref] [PubMed]
- Han M, Brannigan RE, Antenor JA, et al. Association of hemospermia with prostate cancer. J Urol 2004;172:2189-92. [Crossref] [PubMed]
- Kumar P, Kapoor S, Nargund V. Haematospermia - a systematic review. Ann R Coll Surg Engl 2006;88:339-42. [Crossref] [PubMed]
- Ahmad I, Krishna NS. Hemospermia. J Urol 2007;177:1613-8. [Crossref] [PubMed]
- Lowell DM, Lewis EL. Melanospermia: a hitherto undescribed entity. J Urol 1966;95:407-11. [PubMed]
- Smith GW, Griffith DP, Pranke DW. Melanospermia: an unusual presentation of malignant melanoma. J Urol 1973;110:314-6. [PubMed]
- Bhaduri S, Riley VC. Haematospermia associated with malignant hypertension. Sex Transm Infect 1999;75:200. [Crossref] [PubMed]
- Close CF, Yeo WW, Ramsay LE. The association between haemospermia and severe hypertension. Postgrad Med J 1991;67:157-8. [Crossref] [PubMed]
- Munkel witz R, Krasnokutsky S, Lie J, et al. Current perspectives on hematospermia: a review. J Androl 1997;18:6-14.
- Akhter W, Khan F, Chinegwundoh F. Should every patient with hematospermia be investigated? A critical review. Cent European J Urol 2013;66:79-82. [Crossref] [PubMed]
- Polito M, Giannubilo W, d'Anzeo G, et al. Hematospermia: diagnosis and treatment. Arch Ital Urol Androl 2006;78:82-5. [PubMed]
- Bamberger E, Madeb R, Steinberg J, et al. Detection of sexually transmitted pathogens in patients with hematospermia. Isr Med Assoc J 2005;7:224-7. [PubMed]
- Gualdi GF, Volpe A, Polettini E, et al. Comparison of magnetic resonance and other imaging methods in the diagnosis of diseases of the seminal vesicles. Clin Ter 1993;142:369-73. [PubMed]
- Worischeck JH, Parra RO. Chronic hematospermia: assessment by transrectal ultrasound. Urology 1994;43:515-20. [Crossref] [PubMed]
- Yagci C, Kupeli S, Tok C, et al. Efficacy of transrectal ultrasonography in the evaluation of hematospermia. Clin Imaging 2004;28:286-90. [Crossref] [PubMed]
- Etherington RJ, Clements R, Griffiths GJ, et al. Transrectal ultrasound in the investigation of haemospermia. Clin Radiol 1990;41:175-7. [Crossref] [PubMed]
- Zhao H, Luo J, Wang D, et al. The value of transrectal ultrasound in the diagnosis of hematospermia in a large cohort of patients. J Androl 2012;33:897-903. [Crossref] [PubMed]
- Furuya S, Ogura H, Saitoh N, et al. Hematospermia: an investigation of the bleeding site and underlying lesions. Int J Urol 1999;6:539-47; discussion 548. [Crossref] [PubMed]
- Raviv G, Laufer M, Miki H. Hematospermia--the added value of transrectal ultrasound to clinical evaluation: is transrectal ultrasound necessary for evaluation of hematospermia? Clin Imaging 2013;37:913-6. [Crossref] [PubMed]
- Lencioni R, Ortori S, Cioni D, et al. Endorectal coil MR imaging findings in hemospermia. MAGMA 1999;8:91-7. [Crossref] [PubMed]
- Cho IR, Lee MS, Rha KH, et al. Magnetic resonance imaging in hemospermia. J Urol 1997;157:258-62. [Crossref] [PubMed]
- Furuya S, Furuya R, Masumori N, et al. Magnetic resonance imaging is accurate to detect bleeding in the seminal vesicles in patients with hemospermia. Urology 2008;72:838-42. [Crossref] [PubMed]
- Prando A. Endorectal magnetic resonance imaging in persistent hemospermia.
- Li YF, Liang PH, Sun ZY, et al. Imaging diagnosis, transurethral endoscopic observation, and management of 43 cases of persistent and refractory hematospermia. J Androl 2012;33:906-16. [Crossref] [PubMed]
- Okubo K, Maekawa S, Aoki Y, et al. In vivo endoscopy of the seminal vesicle. J Urol 1998;159:2069-70. [Crossref] [PubMed]
- Song Y, Zhao J, Dong Y. Application of the Ureteroscope for Diagnosis and Treatment of the Seminal Vesicle Diseases. Int Surg 2015;100:1233-6. [Crossref] [PubMed]
- Liu B, Li J, Li P, et al. Transurethral seminal vesiculoscopy in the diagnosis and treatment of intractable seminal vesiculitis. J Int Med Res 2014;42:236-42. [Crossref] [PubMed]
- Cui ZQ, Wang YC, Du J, et al. Transurethral seminal vesiculoscopy combined with finasteride for recurrent hematospermia. Zhonghua Nan Ke Xue 2014;20:536-8. [PubMed]
- Wang L, Liu ZY, Xu CL, et al. Transurethral seminal vesiculoscopy for refractory or recurrent hemospermia: clinical analysis of 162 cases. Zhonghua Nan Ke Xue 2013;19:531-4. [PubMed]
- Fu FW, Che JP, Gao Y, et al. Transurethral ureteroscopy in the diagnosis and treatment of hemospermia. Zhonghua Nan Ke Xue 2010;16:1105-7. [PubMed]
- Liu ZY, Sun YH, Xu CL, et al. Transurethral seminal vesiculoscopy in the diagnosis and treatment of persistent or recurrent hemospermia: a single-institution experience. Asian J Androl 2009;11:566-70. [Crossref] [PubMed]
- Xing C, Zhou X, Xin L, et al. Prospective trial comparing transrectal ultrasonography and transurethral seminal vesiculoscopy for persistent hematospermia. Int J Urol 2012;19:437-42. [Crossref] [PubMed]