Ejaculatory dysfunction in the treatment of lower urinary tract symptoms
Introduction
The link between lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) and sexual dysfunction is well established. Sexual dysfunction can encompass both ejaculatory dysfunction (EjD) and erectile dysfunction (ED). Ejaculatory dysfunction can consist of premature ejaculation, delayed ejaculation, retrograde ejaculation, anejaculation, decreased force of ejaculation and pain upon ejaculation. The Multinational Study on the Aging Male (MSAM-7) has firmly established an epidemiological relationship between worsening LUTS and both ED/EjD (1).
Treatment for LUTS includes observation, medical management, and surgical therapy. Either medical management or surgical therapy is recommended for those with moderate to severe LUTS both to improve quality of life and reduce the risk of disease progression. Unfortunately both options increase the risk of EjD. The risk of EjD with medical management depends upon which agent is used. Alpha blockers frequently lead to anejaculation. The addition of a 5-α reductase inhibitors (5ARI) further increases the risk of EjD. The incidence of EjD following surgical therapy is highly dependent upon which intervention is chosen. The current gold standard, transurethral resection of the prostate (TURP), causes EjD in 65% of patients and most urologist appropriately counsel patients accordingly (2). Less invasive therapies may spare ejaculatory function but at the cost of reduced efficacy in relieving LUTS. This review will survey the relationship between LUTS/BPH and EjD. The impact of different medical and surgical therapies on ejaculatory function will be reviewed.
Prevalence of LUTS/EjD in the general population
Multiple large scale studies have established the epidemiologic relationship between LUTS and EjD. The MSAM-7, a large scale multinational study involving six European countries and the USA, demonstrated that the prevalence of overall EjD was greater than 90% with 5% of respondents reporting the complete absence of ejaculation. In this study it appears that moderate-to severe EjD symptoms increase with age with ejaculatory complaints in 30.1%, 54.9%, and 74.3% of men 50–59, 60–69, 70–80 respectively (1). A recent analysis by Wein et al. suggested that both EjD and premature ejaculation are increased in patients with multiple LUTS (3). Multiple studies with diverse geographic locations have firmly established the link between LUTS and EjD (Table 1).
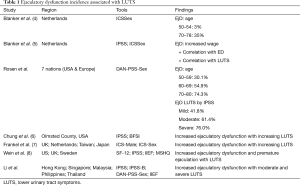
Full table
Pathophysiology of ejaculatory function
Few studies have investigated the pathophysiology linking BPH to EjD. Most research efforts have been clinical studies exploring the relationship between the medical and surgical treatment of BPH with EjD. Currently the basic understanding of the pathophysiology is limited. Ejaculation is a complex process, composed of two main phases, emission (ejaculate is deposited into the prostatic urethra from testes and accessory sex glands) and expulsion (semen is forcefully expelled from the urethral meatus). The reflex involves an interplay of somatic, sympathetic, and parasympathetic pathways (9).
Pathways related to endothelial dysfunction have been implicated as being involved in both the development of LUTS as well EjD. The pathophysiologic mechanisms discussed in the literature include: reduced signaling in the nitric oxide/cGMP pathway, increased RhoA-Rho-kinase (ROCK) signaling, autonomic hyperactivity, and pelvic atherosclerosis with associated pelvic ischemia (10,11). Nitric oxide synthase (NOS) containing neurons have been identified in the smooth muscle, urothelium, blood vessels, and bladder. Their highest concentration is at the region around the bladder neck and bladder outlet (12). NOS activity is necessary for the production of nitric oxide and therefore formation of cGMP, which mediates smooth muscle relaxation by decreasing intracellular levels of calcium. RhoA-Rho-kinase (ROCK), a mediator of α-adrenergic and endothelin-1 (ET-1) triggered smooth muscle contraction, is another important player in maintaining proper smooth muscle tone in the LUT. An up-regulated RhoA/ROCK pathway could impair smooth muscle relaxation, and be involved in LUTS and EjD. Since normal ejaculatory function, particularly the emission phase, relies on the proper balance between sympathetic and parasympathetic nervous activity (13), any condition which disturbs the balance should be investigated for its role in EjD. Autonomic hyperactivity (AH), a component of metabolic syndrome, is a dysregulation between the sympathetic and parasympathetic tone. AH has been implicated as having a role in the development of LUTS as well as causing subjective dysfunctional voiding (14). Pelvic atherosclerosis reduces NO signaling, up regulates RhoA-ROCK contractile signaling, and is a component of metabolic syndrome and autonomic hyperactivity. Therefore, atherosclerosis of the lower urinary tract, particularly the prostate, penis and bladder, is regarded as the mechanism responsible for tying together all the theories previously described (15).
EjD, defined as any disturbance in ejaculation, entails a wide array of disorders, each with a potentially different mechanism related to LUTS. Retrograde ejaculation is the result of a problem during the expulsion phase of ejaculation. Emission of semen into the prostatic urethra remains intact. However, during expulsion, semen is sent into the urinary bladder, rather than being sent antegrade into the penile urethra. The retrograde flow of ejaculate into the bladder is permitted by a pathologically open internal vesical sphincter, or bladder neck (16). Endothelial dysfunction that leads to irregular NOS signaling and smooth muscle relaxation at the bladder neck/outlet could play a major role in the development of retrograde ejaculation and LUTS. Anejaculation, the complete loss of ejaculation, and decreased volume of ejaculate could be caused by decreased force of LUT smooth muscle contraction. Decreased secretions from the prostate gland, seminal vesicle, testis, or epididymis also play a role in anejaculation. One understood cause of painful ejaculation is aberrant sensation and inflammation from the prostate, which is also a known cause of LUTS. As will be explored subsequently many of the medical treatments for BPH are a cause of anejaculation.
Effect of BPH treatments on ejaculatory function
Surgery
TURP
It is well established that TURP causes ejaculatory dysfunction although it remains the gold standard for the treatment of BPH. The AUA BPH guideline panel estimates the incidence of of EjD with TURP at 65% (2). Consistent with this a review of 30 RCTs involving TURP showed the rate of EjD at 66.1% (8). It must be appreciated however that pre-operative ejaculatory dysfunction is common in men undergoing TURP being estimated around 30–35%. In this CLasP study, which randomized men to either watchful waiting, TURP, On Nd: YAG resection, the rates of EJD were 43%, 62%, and 65% respectively. It is interesting to note that the rates of ejaculatory pain were decreased post-operatively in men undergoing TURP or Nd:YAG resection (17). A trial comparing monopolar versus bipolar TURP did not show a difference in the rate of ejaculatory dysfunction although the study was likely not sufficiently powered to detect a difference (18). The volume of resection does not appear to impact the incidence of EjD (19).
HOLEP
Holmium laser emits a 2,140 nm wavelength, preferentially absorbed by water which can be used to enucleate and/or resect prostatic adenoma. A RCT by Kuntz et al. with 200 men comparing HOLEP with TURP showed very similar rates of EjD at 74.0% and 70.3% respectively (20). A smaller study by Wilson et al. with 61 patients with prostates larger than 40 grams showed similar rates of EjD in the small number of men sexually active 75.0% for HOLEP versus 61.5% for TURP (21). A recent pilot study explored a technique of sparing tissue within 1 cm of the verumontanum. Twenty-six patients underwent conventional HOLEP and 26 underwent an ejaculatory sparing HOLEP. The ejaculatory sparing technique did not dramatically reduce the rate of EjD. Of patients who underwent conventional HOLEP none had any ejaculatory function while only 15% in the ejaculatory sparing technique avoided EjD (22). Clearly these modifications of standard procedures will require additional study before any endorsement can be made.
Photovaporization
PVP vaporization uses a light wavelength between 53 and 1,064 nm which is preferentially absorbed by hemoglobin over water leading to rapid vaporization of prostatic tissue (2). The GOLIATH study, a multicenter randomized trial, compared the Greenlight XPS to TURP. Greenlight XPS was noninferior to TURP for IPSS improvement, Qmax, and complications. The study reported EjD as an adverse event with similar rates of 67.1% and 65.1% for Greenlight PVP and TURP respectively (23).
Transurethral microwave therapy (TUMT)/transurethral needle ablation (TUNA)
TUMT and TUNA results in lower rates of EjD than TURP. The studies examining the difference are reporting EjD as an adverse event and it is not assessed by validated questionaires. TUMT used both radiating heat energy and conductive cooling to induce damage of the lateral lobes while sparing the urethra. Francisca et al. conducted a randomized trial in 147 patients comparing TUMT to TURP. At 3 months 74% of those who underwent TUMT had preserved antegrade ejaculation with 27% in the TURP group (24). Likewise another randomized study comparing TUMT and TURP showed a similar difference in rates of antegrade ejaculation (77.8% vs. 36.9%). In the latter study, consistent with the bulk of the literature, TUMT did not match TURP for objective improvement in LUTS (25).
Urolift
The prostatic urethral lift, Urolift, is a tissue sparing approach which involves the use of implants placed under cystoscopic guidance to retract the obstructing lateral lobes of the prostate. Data thus far indicate that the Urolift demonstrates a modest improvement in ejaculatory function with this treatment. The implant consists of a nitinol capsular tab, an adjustable polyeytheleterephthalate filament, and a stainless steel urethral piece which epithelializes. Prospectively collected data from the L.I.F.T. study using the MSQH-EjD questionnaire showed improvement in EjD in patients undergoing Urolift and reduced bother at one year of follow up (26). A recent review of this data it appears that at the 3-year mark the confidence interval (CI) crosses 0 suggesting that improvement in EjD may be transient (27).
Convective water vapor energy ablation
The recently introduced Rezum System (NxThera, Inc., Maple Grove, MN) uses convective water vapor energy (WAVE™) as a thermal therapy to the transition zone of the prostate. This wet thermal energy in the form of water vapor that is dispersed into the prostatic adenoma which subsequently undergoes cell death and necrosis. No thermal effect occurs outside of the prostate. The initial trial by McVary et al. showed greater improvements in IPSS, Qmax, and peak flow rate in those who underwent thermal therapy as opposed to a sham procedure. No de novo erectile dysfunction was reported in the treatment group. Anejaculation, reported as an adverse event occurs in 2.9% of those who had thermal therapy and 0% in the sham group (28). In this RCT the IIEF and MSHQ-EjD-function score were not different from control at 3 months or from baseline at 1 year. The ejaculatory bother score improved 31% over baseline (P=0.0011). Also, 32% of subjects achieved minimal clinically important differences (MCID) in EF scores at 3 months, and 27% at 1 year including those with moderate to severe erectile dysfunction (ED). IPSS and Qmax were significantly superior to controls at 3 months and throughout 1 year (P<0.0001) (29).
Medical therapies
Alpha-blockers
Alpha adrenoreceptor antagonists are a mainstay in the treatment of LUTS associated with BPH. α1-adrenoceptors are stimulated physiologically by catecholamines. Catecholamines act on α1-adrenorectors on vascular smooth muscle to increase vascular tone and raise blood pressure. Additionally, these agents act on the smooth muscle in the prostate and bladder neck. Nonselective agents (terazosin, doxazosin) are associated with significant postural hypotension. Alfuzosin is also a nonselective agent although it does have lower rates of hypotension compared to terazosin and doxazosin. Tamsulosin and silodosin are α1a receptor antagonist associated with lower rates of postural hypotension but higher rates of ejaculatory dysfunction secondary to the predominance of α1a receptors in the vas deferens and seminal vesicles (30).
Many studies label the ejaculatory dysfunction associated with alpha blockers as retrograde ejaculation. Although this term is used in many of the large studies on the treatment of LUTS in BPH patients it actually represents anejaculation. Animal studies using knockout mice for the α1A adrenoreceptor have shown decreased rates of pregnancy and decreased contraction of the vas deferens in response to norepinephrine (31). In a study of 15 healthy male volunteers in a double-blind crossover trial all participants reported the complete absence of ejaculation. Post masturbation urinalysis did not demonstrate notable levels of sperm in any of the patients consistent with anejaculation rather than retrograde ejaculation (32). A double blind trial comparing tamsulosin, alfuzosin and placebo by Hellstrom et al. showed similar levels of semen in post-ejaculatory urinalysis, thus supporting the hypothesis that alpha blockers induce anejaculation not retrograde ejaculation (33).
Alfuzosin
Alfuzosin is a nonselective alpha adrenoreceptor antagonist with equal affinity for α1a-, α1b-, and α1d subtypes but rarely causes hypotension (34). It does not enter the central nervous system. It is administered as a 10 mg daily dose and does not require titration. Three large double blind, placebo control trials demonstrated the safety and efficacy of alfuzosin for treating LUTS in BPH patients. The incidence of impaired ejaculation was 0.6% versus 0% of those taking placebo (35). An open label extension of one of the double blind trials showed that the incidence of ejaculatory dysfunction did not increase with one year of follow up (36). In fact a one year observation study of men treated with alfuzosin for LUTS reported improvements in amount of ejaculate and reduced pain/discomfort associated with ejaculation (37). A randomized, double-blind, placebo-controlled, 3-way crossover design, with healthy male volunteers by Hellstrom et al. also showed that tamsulosin negatively impacts semen parameters (33).
Doxazosin/terazosin
These alpha adrenoreceptor antagonists were used in the treatment of hypertension before being used for the treatment of LUTS/BPH. Both these agents show equal affinity for α1a-, α1b-, and α1d receptor subtypes; therefore, these agents are associated with higher rates of asthenia and dizziness than placebo. Consistent with its nonselective features multiple trials have demonstrated that these agents have no higher rate of ejaculatory dysfunction compared with placebo (38,39).
Silodosin
Silodosin is a selective α1a adrenoreceptor antagonist approved for the treatment of BPH. It has the highest selectivity for the α1a versus α1b (162:1 ration) receptor of the commercially used alpha blockers (40). A large phase III trial in Europe demonstrated equal efficacy with tamsulosin for LUTS associated with BPH (41). During the 12 weeks of follow up with this study those taking silodosin had a higher rate of ejaculatory dysfunction than those taking tamsulosin (14.2% vs. 2.1%). An observational study of 30 men with moderate to severe LUTS who took silodosin 8 mg/daily reported a 90% prevalence of impaired ejaculation during the 4 weeks of follow up. Additional TRUS imaging of the seminal vesicles demonstrated a mean doubling of their volume after four weeks of therapy (8.1±6.4 vs. 16.4±8.2 cc) (42). There are conflicting studies whether orgasmic function is impaired in patients taking silodosin. A study out of Japan examined the effect of 4 mg daily silodosin on 15 healthy volunteers. One hundred percent reported anejaculation. All participants reported having an orgasm with although 80% reported that this was either somewhat uncomfortable or unsatisfying (43).
Tamsulosin
Tamsulosin is a selective α1-adrenoreceptor antagonist used in the treatment of BPH which has affinity for α1A over α1B. Tamsulosin also acts on dopaminergic and serotinergic receptors in the CNS (44). α1A adrenoreceptors are distributed in the epididymis, vas deferens, seminal vesicles, prostate, and bladder neck, all of which are involved in the emission phase of ejaculation. The seminal vesicles contribute approximately 80% of the ejaculate volume therefore blockade of α1A adrenoreceptors leads to anejaculation. Data from the AUA BPH Guideline panel shows a median incidence of EjD of 10% in those treated with tamsulosin (45). The duration of therapy does appear to impact the incidence of EjD. One open label study extension study with tamsulosin (follow up duration 77–104 weeks) the incidence of abnormal ejaculation was 30%.
Animal studies have shown that the effects of tamsulosin and alfuzosin on the bladder neck and seminal vesicles are similar (46). This raises the likelihood that the pronounced effects of tamsulosin on ejaculation are at least in part due to effects on the CNS. This is consistent with studies showing intravenous administration of tamsulosin impaired contraction of the bulbospongiosus muscle while alfuzosin did not (47).
5-alpha-reductase inhibitors
Inhibition of the enzyme 5-alpha reductase, which catalyzes the conversion of testosterone to dihydrotestosterone is another medical therapy used for the treatment of BPH/LUTS. The type 1 isoenzyme is expressed in most tissues while the type 2 isoenzymes is primarily within the male reproductive tract. Finasteride selectively inhibits the type 2 isoenzyme. Dutasteride inhbits both the type 1 and type 2 isoenzymes. Those taking finasteride have a 4% adverse event rate of EjD compared to 1% in those taking placebo (30). A multicenter double blind RCT comparing dutasteride to placebo demonstrated a 2.2% and 0.8% incidence of EjD as an adverse event (48). The prevalence of EjD appears to decrease after one year of treatment. The mechanism of this adverse effect is not completely understood. Fwu et al. analyzed prospectively collected data on EjD from the EjD using the BMSFI questionnaire. This study found a small but statistically significant increase in EjD in men taking finasteride with 18% reporting worsening of ejaculatory function versus 12% in those taking placebo at four years of follow-up (49).
In 2011 the FDA took an interest in adverse events (AEs) related to 5ARI exposure and began collecting information on all 5ARIs using the adverse event reporting system (FAERS) (50). Meanwhile, the National Institutes of Health sent out a Global Public Health Advisory recognizing a cluster of symptoms that are associated with finasteride: Post-Finasteride Syndrome (PFS). A small percentage of men on finasteride 1 mg complained of erectile dysfunction, change of libido or semen quality compared to placebo (3.8% vs. 2.1%) (51). Some studies with questionable methodology noted the 1mg dose for male pattern baldness (MPB) was associated with persistent symptoms (52,53). Although there was no conclusive evidence regarding AEs seen with finasteride 5 mg (versus 1 mg finasteride), the FDA mandated a label change on all finasteride products advising a risk of libido loss, erectile dysfunction, ejaculatory disorders, gynecomastia, and other adverse sexual experiences. Shortly thereafter, the company that makes dutasteride voluntarily changed their product label, presumably with the thought that the AEs surrounding finasteride would apply to all 5ARI’s. In a recent review of the FDA database, dutasteride was not implicated for PFS. The reported persistent nature of AE associated with finasteride or whether or not PFS is real or imagined cannot be assessed from the FAERS database. PFS requires further research utilizing a matched control group that shares baseline characteristics of those reporting PFS.
Combination therapy (5 ARI with alpha blocker)
The sexual side effects associated with combined therapy consisting of an alpha blocker and a 5ARI are qualitatively them same but with a higher incidence in those on combination compared to monotherapy. The MTOPS study compared finasteride/doxazosin monotherapy, monotherapy with finasteride, monotherapy with doxazosin, to placebo. The rates of EjD were 14.1% for combination therapy, 7.2% for finasteride monotherapy, 4.5% for doxazosin monotherapy, and 2.3% for placebo (54). The COMBAT trial was a large multicenter RCT comparing tamsulosin monotherapy, dutasteride monotherapy, and tamsulosin/dutasteride combination therapy. There was no placebo arm. This study also demonstrated a higher rate of EjD with combination therapy than monotherapy (55).
PDE5I and anti-muscarinics
PDE5I are first line treatment for use in erectile dysfunction. These agents have been increasingly utilized for the treatment of LUTS/BPH. By increasing cellular levels of cGMP these medications have been shown to increase LUT oxygenation, induce smooth muscle relaxation in the prostate and bladder neck, decrease prostatic stromal proliferation, and reduce afferent nerve activity from the LUT (56). These agents have been shown to reduce prostatic inflammation, particularly in men with the metabolic syndrome (57,58). When used to treatment men with BPH, PDE5Is have shown improved IPSS scores, improved QOL scores with minimal effect on maximum urinary flow rate (59,60). While PDE5Is have been used for premature ejaculation, particularly in conjunction with SSRIs, at the current time there is no data measuring their impact upon ejaculatory function in patients with BPH (61).
Antimuscarinics are used in the treatment of both male and female OAB symptoms. There are five muscarinic receptor types (M1, M2, M3, M4 and M5). M2 and M3 are the primary receptors in the male LUT (62). Animal studies suggests that the M3 receptor is active in both the vas deferens and seminal vesicles (63,64). At this time there is no data clarifying the impact that anticholinergic medications may have on ejaculatory function.
Phytotherapy
Phytotherapeutic agents are frequently taken for the treatment of LUTS/BPH. They are derived from the roots, seed, bark, and fruits of plants (Table 2). They are generally well tolerated. Overall there is little data addressing the impact of phyotherapeutic agents for EjD. In a randomized trial comparing Serenoa Repens with Tamsulosin the rate of EjD was higher with Tamsulson (4.2% vs. 0.6%) (65).

Full table
Conclusions
Both EjD and BPH are very common disorders in men under the care of an urologist. It is well documented that there is a clinical association between these two entities. Unfortunately many of the medical treatments and almost all surgical treatment impact the ejaculatory function of the patient. The surgical treatment of BPH often leads to retrograde ejaculation while medical treatment leads to anejaculation. Selective α-blockers, tamsulosin and silodosin are more likely to be associated with EjD although they tend to be better tolerated. Alfuzosin, doxazosin, and terazosin rarely cause EjD. The most effective surgical therapies for BPH (TURP, HoLEP, Photovaporization) tend to cause highest rates of EjD despite overall QOL scores being higher postoperatively. Patients should be thoroughly counseled regarding the ejaculatory impact any BPH therapy may have.
Acknowledgements
This work was supported by the SIU Urology Research Endowment fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol 2003;44:637-49. [Crossref] [PubMed]
- AUA Practice Guidelines Committee for the American Urological Association. AUA guideline on management of benign prostatic hyperplasia (2003)—Chapter 3: Results of the treatment outcomes analyses. Linthincum, MD: American Urological Association, 2003.
- Wein AJ, Coyne KS, Tubaro A, et al. The impact of lower urinary tract symptoms on male sexual health: EpiLUTS. BJU Int 2009;103 Suppl 3:33-41. [Crossref] [PubMed]
- Blanker MH, Bosch JL, Groeneveld FP, et al. Erectile and ejaculatory dysfunction in a community-based sample of men 50 to 78 years old: prevalence, concern, and relation to sexual activity. Urology 2001;57:763-8. [Crossref] [PubMed]
- Blanker MH, Bohnen AM, Groeneveld FP, et al. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community-based study. J Am Geriatr Soc 2001;49:436-42. [Crossref] [PubMed]
- Chung WS, Nehra A, Jacobson DJ, et al. Lower urinary tract symptoms and sexual dysfunction in community-dwelling men. Mayo Clin Proc 2004;79:745-9. [Crossref] [PubMed]
- Frankel SJ, Donovan JL, Peters TI, et al. Sexual dysfunction in men with lower urinary tract symptoms. J Clin Epidemiol 1998;51:677-85. [Crossref] [PubMed]
- Marra G, Sturch P, Oderda M, et al. Systematic review of lower urinary tract symptoms/benign prostatic hyperplasia surgical treatments on men's ejaculatory function: Time for a bespoke approach? Int J Urol 2016;23:22-35. [Crossref] [PubMed]
- Hellstrom WJ, Giuliano F, Rosen RC. Ejaculatory dysfunction and its association with lower urinary tract symptoms of benign prostatic hyperplasia and BPH treatment. Urology 2009;74:15-21. [Crossref] [PubMed]
- Gacci M, Eardley I, Giuliano F, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 2011;60:809-25. [Crossref] [PubMed]
- McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int 2006;97:23-28; discussion 44-5. [Crossref] [PubMed]
- Hedlund P. Nitric oxide/cGMP-mediated effects in the outflow region of the lower urinary tract--is there a basis for pharmacological targeting of cGMP? World J Urol 2005;23:362-7. [Crossref] [PubMed]
- Hellstrom WJ, Giuliano F, Rosen RC. Ejaculatory dysfunction and its association with lower urinary tract symptoms of benign prostatic hyperplasia and BPH treatment. Urology 2009;74:15-21. [Crossref] [PubMed]
- McVary KT, Rademaker A, Lloyd GL, et al. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2005;174:1327-433. [Crossref] [PubMed]
- Moul S, McVary KT. Lower urinary tract symptoms, obesity and the metabolic syndrome. Curr Opin Urol 2010;20:7-12. [Crossref] [PubMed]
- Fedder J, Kaspersen MD, Brandslund I, et al. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology 2013;1:602-6. [Crossref] [PubMed]
- Donovan JL, Peters TJ, Neal DE, et al. A randomized trial comparing transurethralresection of the prostate, laser therapy and conservative treatment of men with symptoms associated with benign prostatic enlargement: the CLasP study. J Urol 2000;164:65-70. [Crossref] [PubMed]
- Chen Q, Zhang L, Fan QL, et al. Bipolar transurethral resection in saline vs traditional monopolar resection of the prostate: results of a randomized trial with a 2-year follow-up. BJU Int 2010;106:1339-43. [Crossref] [PubMed]
- Møller-Nielsen C, Lundhus E, Moller-Madsen B, et al. Sexual life following“minimal” and “total” transurethral prostatic resection. Urol Int 1985;40:3-4. [Crossref] [PubMed]
- Kuntz RM, Ahyai S, Lehrich K, et al. Transurethral holmium laser enucleation of the prostate versus transurethral electrocautery resection of the prostate: a randomized prospective trial in 200 patients. J Urol 2004;172:1012-6. [Crossref] [PubMed]
- Wilson LC, Gilling PJ, Williams A, et al. A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40grams: results at 2 years. Eur Urol 2006;50:569-73. [Crossref] [PubMed]
- Kim M, Song SH, Ku JH, et al. Pilot study of the clinical efficacy of ejaculatory hood sparing technique for ejaculation preservation in Holmium laser enucleation of the prostate. Int J Impot Res 2015;27:20-4. [Crossref] [PubMed]
- Bachmann A, Tubaro A, Barber N, et al. 180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial—the GOLIATH study. Eur Urol 2014;65:931-42. [Crossref] [PubMed]
- Francisca EA, d'Ancona FC, Meuleman EJ, et al. Sexual function following high energy microwave thermotherapy: results of a randomized controlled study comparing transurethral microwave thermotherapy to transurethral prostatic resection. J Urol 1999;161:486-90. [Crossref] [PubMed]
- Ahmed M, Bell T, Lawrence WT, et al. Transurethral microwave thermotherapy (Prostatron version 2.5) compared with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a randomized, controlled, parallel study. Br J Urol 1997;79:181-5. [Crossref] [PubMed]
- McVary KT, Gange SN, Shore ND, et al. Treatment of LUTS secondary to BPH while preserving sexual function: randomized controlled study of prostatic urethral lift. J Sex Med 2014;11:279-87. [Crossref] [PubMed]
- Roehrborn CG, Rukstalis DB, Barkin J, et al. Three year results of the prostatic urethral LIFT study. Can J Urol 2015;22:7772-82. [PubMed]
- McVary KT, Gange SN, Gittelman MC, et al. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J Urol 2016;195:1529-38. [Crossref] [PubMed]
- McVary KT, Gange SN, Gittelman MC, et al. Erectile and Ejaculatory Function Preserved With Convective Water Vapor Energy Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Randomized Controlled Study. J Sex Med 2016;13:924-33. [Crossref] [PubMed]
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011;185:1793-803. [Crossref] [PubMed]
- Sanbe A, Tanaka Y, Fujiwara Y, et al. β1-Adrenoceptors are required for normal male sexual function. Br J Pharmacol 2007;152:332-40. [Crossref] [PubMed]
- Kobayashi K, Masumori N, Hisasue S, et al. Inhibition of Seminal Emission Is the Main Cause of Anejaculation Induced by a New Highly Selective ly -Blocker in Normal Volunteers. J Sex Med 2008;5:2185-90. [Crossref] [PubMed]
- Hellstrom WJ, Sikka SC. Effects of Alfuzosin and Tamsulosin on Sperm Parameters in Healthy Men: Results of a Short-Term, Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J Androl 2009;30:469-74. [Crossref] [PubMed]
- Mottet N, Bressolle F, Delmas V, et al. Prostatic tissual distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur Urol 2003;44:101-5. [Crossref] [PubMed]
- Roehrborn CG, Van Kerrebroeck P, Nordling J. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. BJU Int 2003;92:257-61. [Crossref] [PubMed]
- van Kerrebroec P, Jardin A, van Cangh P, et al. Long-term safety and efficacy of a once-daily formulation of alfuzosin 10 mg in patients with symptomatic benign prostatic hyperplasia: open-label extension study. Eur Urol 2002;41:54-60; discussion 60-1. [Crossref] [PubMed]
- van Moorselaar RJ, Hartung R, Emberton M, et al. Alfuzosin 10 mg once daily improves sexual function in men with lower urinary tract symptoms and concomitant sexual dysfunction. BJU Int 2005;95:603-8. [Crossref] [PubMed]
- Cardura XL (doxazosin mesylate) [prescribing information]. New York: Pfizer Inc., 2006. Available online: http://labeling.pfizer.com/showlabeling.aspx?id=535, June 22, 2016.
- Hytrin (terazosin HCl) capsules [prescribing information]. North Chicago, IL: Abbott Laboratories, 2001. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019057s022lbl.pdf, June 22, 2016.
- Tatemichi S, Kobayashi K, Maezawa A, et al. Alpha1-adrenoceptor subtype selectivity and organ specificity of silodosin (KMD-3213). Yakugaku Zasshi 2006;126:209-16. [Crossref] [PubMed]
- Chapple CR, Montorsi F, Tammela TL, et al. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo-and active-controlled clinical trial performed in Europe. Eur Urol 2011;59:342-52. [Crossref] [PubMed]
- Bozkurt O, Demir O, Sen V, et al. Silodosin causes impaired ejaculation and enlargement of seminal vesicles in sexually active men treated for lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology 2015;85:1085-9. [Crossref] [PubMed]
- Kobayashi K, Masumori N, Kato R, et al. Orgasm is preserved regardless of ejaculatory dysfunction with selective β1A-blocker administration. Int J Impot Res 2009;21:306-10. [Crossref] [PubMed]
- Andersson KE, Wyllie MG. Ejaculatory dysfunction: why allβ-blockers are not equal. BJU Int 2003;92:876-7. [Crossref] [PubMed]
- Giuliano F, Bernabe J, Droupy S, et al. A comparison of the effects of tamsulosin and alfuzosin on neurally evoked increases in bladder neck and seminal vesicle pressure in rats. BJU Int 2004;93:605-8. [Crossref] [PubMed]
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387-98. [Crossref] [PubMed]
- Giuliano FA, Clément P, Denys P, et al. Comparison between tamsulosin and alfuzosin on the expulsion phase of ejaculation in rats. BJU Int 2006;98:876-9. [Crossref] [PubMed]
- Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002;60:434-41. [Crossref] [PubMed]
- Fwu CW, Eggers PW, Kirkali Z, et al. Change in sexual function in men with lower urinary tract symptoms/benign prostatic hyperplasia associated with long-term treatment with doxazosin, finasteride and combined therapy. J Urol 2014;191:1828-34. [Crossref] [PubMed]
- Available online: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm299754.htm
- McClellan KJ, Markham A. Finesteride. A review of its use in male pattern hair loss. Drugs 1999;57:111-26. [Crossref] [PubMed]
- Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med 2011;8:1747-53. [Crossref] [PubMed]
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol 2010;146:1141-50. [Crossref] [PubMed]
- Fwu CW, Eggers PW, Kirkali Z, et al. Change in sexual function in men with lower urinary tract symptoms/benign prostatic hyperplasia associated with long-term treatment with doxazosin, finasteride and combined therapy. J Urol 2014;191:1828-34. [Crossref] [PubMed]
- Roehrborn CG, Siami P, Barkin J, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 2008;179:616-21. [Crossref] [PubMed]
- Gacci M, Andersson KE, Chapple C, et al. Latest Evidence on the Use of Phosphodiesterase Type 5 Inhibitors for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Eur Urol 2016;70:124-33. [Crossref] [PubMed]
- Vignozzi L, Gacci M, Cellai I, et al. PDE5 inhibitors blunt inflammation in human BPH: a potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 2013;73:1391-402. [Crossref] [PubMed]
- Morelli A, Comeglio P, Filippi S, et al. Mechanism of action of phosphodiesterase type 5 inhibition in metabolic syndrome-associated prostate alterations: An experimental study in the rabbit. Prostate 2013;73:428-41. [Crossref] [PubMed]
- Liu L, Zheng S, Han P, et al. Phosphodiesterase-5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia: A systematic review and meta-analysis. Urology 2011;77:123-9. [Crossref] [PubMed]
- Gacci M, Corona G, Salvi M, et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with β-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 2012;61:994-1003. [Crossref] [PubMed]
- Men C, Yu L, Yuan H, et al. Efficacy and safety of phosphodiesterase type 5 inhibitors on primary premature ejaculation in men receiving selective serotonin reuptake inhibitors therapy: a systematic review and meta-analysis. Andrologia 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Tyagi S, Tyagi P, Van-le S, et al. Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol 2006;176:1673-8. [Crossref] [PubMed]
- Hamamura M, Maróstica E, de Avellar MC, et al. Muscarinic acetylcholine receptor subtypes in the rat seminal vesicle. Mol Cell Endocrinol 2006;247:192-8. [Crossref] [PubMed]
- Hsieh JT, Liu SP, Chang HC, et al. Parasympathetic influence plays an independent and significant role in inducing the contraction of the seminal vesicle of the rat. Urology 2010;76:511.e1-4. [Crossref] [PubMed]
- Debruyne F, Koch G, Boyle P, et al. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (tamsulosin) in the treatment of benign prostatic hyperplasia: a 1-year randomized international study. Eur Urol 2002;41:497-506; discussion 506-7. [Crossref] [PubMed]


