Genetics and biological markers in urachal cancer
Introduction
Urachal cancer (UraC) is a rare malignant tumor entity derived from the embryologic structure called urachus. UraCs are mostly adenocarcinomas located at the dome of the bladder, along the mid-line, including the umbilicus and the space of Retzius (between the symphysis pubis and the bladder) (1). The median age of diagnosis is mostly in the fifth and sixth decade, which is on average 10 years earlier than the incidence of urothelial cancer in the bladder (2). The male gender is favored on average 2:1, although literature statistics vary greatly. In Europe, UraC accounts for 0.2% of all bladder cancers and roughly 10–30% of all adenocarcinomas of the bladder (3,4). Due to its embryologic derivation, the definition of UraC incorporates a diversity of cancer entities. Focal glandular metaplasia of the epithelium lining the urachus is believed to create the morphologic basis for the development of intestinal-type adenocarcinomas (1). Other so-called ‘non-glandular urachal-carcinomas’ are recognized as morphologic variants of urachal adenocarcinoma including mucinous, enteric, signet ring cell types or not otherwise specified (NOS) (5). Regarding the different subtypes of adenocarcinoma, no association between prognosis and tumor type could be made so far, due to the paucity of patients (6).
Because of the secondary infiltration of the bladder dome, patients mostly present with delayed symptoms and often advanced disease (7). Symptoms like hematuria (70–80%) and pain (40%), are most commonly described (6-9). Minimal recommended diagnostics include cystoscopy (including biopsies), CT or MRI of the abdomen and a chest X-ray to exclude a metastatic disease (8).
To define the diagnosis of UraC, different sets of criteria have been proposed. Sheldon et al. (7) formulated the most frequently used list of criteria to confirm the diagnosis.
Staging is an important factor in choosing the right treatment option, predicting outcome and ultimately survival when dealing with malignant diseases. For UraC, several staging systems exist next to each other. The Sheldon system, although being criticized for its complexity, remains the most used. Other staging systems, which are less frequently used, are the Mayo system (10) and the TNM system (8) for bladder cancer.
The gold standard for treatment—and so far the only proven cure—is surgical resection including a partial cystectomy. An en-bloc resection of the urachal ligament with the bladder dome and umbilicus is used to appropriately control the tumor (11). Radical cystoprostatectomy is regarded as overtreatment because outcome rates are comparable to partial resection and side effects accumulate (3,10). Most researchers recommend a lymph node dissection if clinical staging showed possible lymph node involvement (11). A higher risk of relapse and a worse prognosis has been reported in cases with positive resection-margins, lymph node involvement, involvement of the peritoneal surface, or when the umbilicus was not resected en-bloc (12). These risk factors may prompt to consider adjuvant chemotherapy for these groups of patients with a higher risk of relapse (2). Local recurrences within the first year are relatively frequent and make up to 30% of higher stage disease (11).
The overall 5-year cancer specific survival rate in the literature differs between 43–61% (13,14). Interestingly, no significant survival difference was found between patients with lymph node metastases compared to those patients with distant organ-metastases (3). Distant metastases have been reported in the visceral organs, the bones and seldomly the brain (7,8). Diffuse local infiltrations in the peritoneal or abdominal wall and distant metastases have a poor prognosis. Patients exhibiting such infiltration can rarely be cured with surgery (3). To date there are no standardized protocols for neo-adjuvant, adjuvant, or salvage chemotherapy regimens for patients who present with metastatic disease (15). Nevertheless, it seems reasonable to treat patients at high risk of relapse with adjuvant chemotherapy (16). So far, adjuvant regimens with combinations, including platinum compounds (cisplatin) and 5-fluorouracil, have shown partial regression of tumor-burden in small cohorts with late metastatic or recurrent disease (17). Alternatively, patients with peritoneal spread might benefit from HIPEC (hyperthermic intraperitoneal chemotherapy) (18,19).
So far the majority of literature consists of small case reports. Overall, UraC is a rare and lethal disease that is often diagnosed in patients at a late stage. It has a heterogeneous histology/biology due to its embryologic derivation. Very few proven treatment options exist next to surgical resection. Many questions regarding the genetic origin, familiarity to other adenocarcinomas, classification, diagnosis, staging and systemic treatment remain unanswered, which is why research on this type of cancer is very valuable.
Materials and methods
For this review, we conducted a literature search in PUBMED investigating the English literature until December 2015. We used “urachal cancer”, “carcinoma of the urachus” and “markers” as keywords. We found 16 useful articles and 2 review articles. In this paper, we provide a comprehensive review of possible markers for this rare disease.
Genetic origin and markers
When choosing an adequate chemotherapeutic regimen, oncologists and pathologists alike find a correlation of urachal tumor with enteric type tumors (16). Sometimes other histologic entities can be the dominant cell type of the UraC. One theory for the etiology of these cells is that their progenitor cells arise from enteric rests, which are left behind from the cloaca during embryologic development. This explains their resemblance with colonic or gastric mucosa (8). Alternatively, metaplasia possibly induced by chronic inflammation may be responsible for the variety of histologic appearances (1,2). Pathologists find that different mucinous subtypes resemble appendiceal, pancreaticobiliary or gynecologic origins (20). Furthermore, signet ring cell types resemble gastric, colorectal or ovarian origins, while a mixture of morphologies and heterogeneity among the different types also occurs (1).
In order to learn more about the genetic origin and look into possible target therapies, genetic research has been conducted. KRAS and BRAF mutations can be detected in colorectal adenocarcinomas, while micro satellite instability (MSI) is associated with mucinous and signet ring cell adenocarcinomas (21). Sirintrapun et al. (22) have looked at KRAS and BRAF mutations and MSI in seven high stages UraC. While BRAF mutations could not be found, KRAS mutations and MSI were present in 6/7 cases. KRAS mutations were associated with a better overall survival in contrast to colorectal adenocarcinomas. Bissonette et al. (5) tried to distinguish the prognostic impact between subtypes of adenomatous UraC using microRNA expression. A variety of microRNAs have been shown to have a significant role as cofactors regulating oncogenes or tumor suppressor genes in carcinogenesis (23). Single histologic subtypes or groups of subtypes failed to show differences in their microRNA expression profiles, leading the authors to suggest that UraC should be viewed as a single biological tumor type. Nakanishi et al. (24) used several techniques on 41 adenomatous UraC to describe their proliferative potential. First DNA quantification status (using Image cytometry, a substitution for the mitotic index) was used, showing a significant relationship for stage and grade. Second, silver nitrate staining of argyrophilic nucleus organizing regions (AgNOR), another technique indication mitotic activity, could show significant higher numbers for mucinous or signet cells. Both techniques failed to predict clinical outcome though. Kipp et al. (25) used Fluorescence in situ hybridization (FISH) to test for chromosomal abnormalities (gain of a single chromosome, polysomy and homozygous 9p21 loss) in rare bladder cancer variants, including adenomatous UraC, using paraffin embedded tissues. The authors found high rates of abnormalities in transitional cell cancer (mean 68%) as well as UraC (mean 45%), concluding that FISH could be an option for the urine diagnostic of rare bladder cancer variants as well.
A very recently presented abstract by Jordan et al. (26) at the ESMO 2015 Conference (European Society for Medical Oncology, Conference), using a gene sequencing assay, shows mutations for adenomatous UraC in Her2 (20%), KRAS (20%) and GNAS (10%) indicating their usefulness for further evaluation. KRAS and GNAS mutations result in the up-regulation of the MAPK signal-transduction pathway, indicating potential for targeted therapy.
Whether the predominant adenomatous tissue in UraC stems from progenitor cells of the cloaca or is due to metaplasia of transitional cells is yet to be revealed. Further genetic studies are needed to look for clinicopathologically relevant mutations that can be used for subtyping or for target treatments.
Immunohistochemical (IHC) markers
In order to form the diagnosis and distinguish UraC from secondary adenocarcinomas, immunohistochemical (IHC) antibodies are being used. Up to date no conclusive marker set has been proposed. Next to the limited case numbers and the embryologic derivation with many subtypes of adenocarcinoma and other non-adenomatous tissues that can be found in UraC, defining a single set of IHC markers remains difficult. A stepwise discriminant analysis of markers for differential diagnosis is necessary (1,27). We have identified four articles that mapped UraC in accordance to other adenocarcinomas (Table 1). In addition two studies using UraC and one study using a mixture of adenocarcinoma of the bladder are presented that evaluate IHC markers as predictors for clinicopathologic characteristics (Table 2).
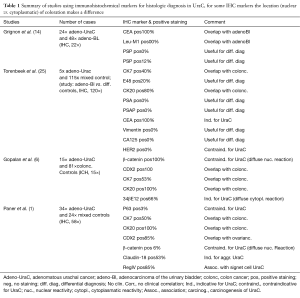
Full table
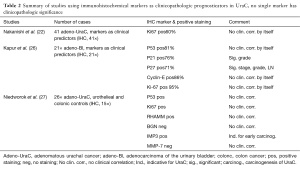
Full table
In general, there is a wide overlap of immunoreactive patterns between IHC markers for adenocarcinomas deriving from enteric organs, the ovaries and the urachus (6). CK7, CK20 and CDX2 stain positive for ductal epithelial found in the colorectal tract (28,29). Three articles agree that CK7, CK20 and CDX2 show an overlapping profile with other adenocarcinomas and are thus useless for UraC if not combined with additional markers (1,6,27). Paner et al. (1) also mapped subtypes and borderline neoplasms. The authors state that signet cell differentiation of UraC is mostly positive for CK7, CK20 and CDX2, compared to colonic or gastric signet cell cancers, which are CK7 negative.
In addition, markers that have been proposed to indicate adenomatous UraC are CEA, 34βE12 (if showing diffuse cytoplasmatic reactivity), both can be found in adenomatous breast cancer, Claudin-18 (in highly aggressive cancers) and RegIV (indicating mucin production and signet cell differentiation). Both Claudin-18 and RegIV are known to stain positive for gastric and pancreatic cancer (30). Argumentative against UraC are Her2, β-catenin (if showing diffuse nuclear reactivity), Leu-M1 and p53. For differential diagnosis of secondary tumors PSA (conclusive for prostatic origin), PSAP, Vimentin (found in sarcomas) and CA125 (indicating ovarian origin) have been used effectively (6,27).
Next to using IHC markers for diagnoses, few authors have looked into the association of biological markers and clinicopathologic characteristics for prognostication. Ki-67, is used as proliferative index marker showing prognostic value for example in bladder cancer (31). Ki-67 as a single marker showed no clinicopathologic relevance (25). In a cohort of 21 adenocarcinomas of the bladder Kapur et al. (32) used a panel of p53, p21, p27, Ki67 and Cyclin E to look into their prognostic value. Cell cycle regulators like p53, p21 and p27 are widely studied and they have prognostic value in urothelial cancer (31). The combined set proved to be prognostic for recurrence and cancer specific mortality. In addition to that p27 and Ki-67 combined were significant for stage, grade, DNA ploidy and lymph node involvement. These findings lead the authors to use these two markers also for urachal adenocarcinomas and in future studies for adenocarcinomas of the bladder. Very recently Niedworok et al. (33) used a panel of p53, Ki-67, RHAMM, BGN, IMP3 and MMP-7 and correlated them with the clinical data of 26 UraC patients. In addition to p53 and Ki-67, RHAMM and IMP3 have prognostic value in urothelial cancer (33,34). Although p53, Ki67, RHAMM, and IMP3 were significantly more positive in UraC compared to urothelial and colonic adenocarcinoma controls, none of the markers were prognostic. The authors mention that IMP3 was remarkably elevated in early stage disease possibly indicating UraC carcinogenesis.
Concluding, the knowledge about IHC markers for UraC is scarce and based mostly on single center or case reports. Still, in a field of overlapping immunoreactivity, several markers have shown promise for differentiating UraC from secondary adenocarcinomas. Further research is necessary to evaluate IHC markers as clinicopathologic predictors and to sub-type UraC. A panel of several markers will be necessary for both these reasons.
Serum markers
Serum markers in general are used as diagnostic tools as well as surveillance markers during or after treatment indicating treatment failure or recurrence. Up to date most case reports are published mentioning markers like CEA, CA19-9, CA125 and CA724 to be elevated in UraC (35-37). CEA and CA19-9 are used in gastrointestinal and pancreatic cancer, while CA125 and CA 724 are useful in ovarian cancer (38,39). In a cohort of 42 UraC Siefker-Radtke et al. (17) reported CEA (59%), CA19-9 (60%) and CA125 (44%) to be elevated. Among those markers CEA was found to mimic the clinical course of patients and their response to therapy the best (17,35,40).
Knowledge about serum markers in UraC is very limited. Currently solely CEA if elevated before treatment can be recommended as useful during follow-up.
Conclusions
The literature on biological markers in UraC is mostly derived from case reports or cohort studies mentioning markers or testing for predictors. Predominantly due to the scarcity of the disease, prospective multi-cohort studies have not been performed. Currently a safety and efficacy study has finished recruitment in North America, looking into the potential of four (5-FU, Leucovorin, Cisplatine, Gemcitabine) chemotherapies for metastatic or unresectable adenocarcinomas including UraC (41). Although this trial will hopefully give insight into some of the questions regarding options and outcome of adjuvant therapy, many basic questions remain unanswered. Genetic research is to show whether UraC stems from progenitor cells of the cloaca or is due to metaplasia of transitional cells. Few IHC markers have shown indicative potential for UraC, a useful panel for differential diagnostics and clinicopathologic prognostication needs yet to be developed. Serum markers show very little potential for neither diagnosis, nor follow-up in UraC. Further research on larger cohorts is necessary to enlarge on potential markers for UraC.
Acknowledgements
Funding: MA Behrendt has received a Sponsorship Grant from the European Sponsorship Program (EUSP, part of the EAU 2015 & 2016).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Paner GP, McKenney JK, Barkan GA, et al. Immunohistochemical analysis in a morphologic spectrum of urachal epithelial neoplasms: diagnostic implications and pitfalls. Am J Surg Pathol 2011;35:787-98. [Crossref] [PubMed]
- Siefker-Radtke A. Urachal adenocarcinoma: a clinician's guide for treatment. Semin Oncol 2012;39:619-24. [Crossref] [PubMed]
- Bruins HM, Visser O, Ploeg M, et al. The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol 2012;188:1102-7. [Crossref] [PubMed]
- Wright JL, Porter MP, Li CI, et al. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer 2006;107:721-8. [Crossref] [PubMed]
- Bissonnette ML, Kocherginsky M, Tretiakova M, et al. The different morphologies of urachal adenocarcinoma do not discriminate genomically by micro-RNA expression profiling. Hum Pathol 2013;44:1605-11. [Crossref] [PubMed]
- Gopalan A, Sharp DS, Fine SW, et al. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol 2009;33:659-68. [Crossref] [PubMed]
- Sheldon CA, Clayman RV, Gonzalez R, et al. Malignant urachal lesions. J Urol 1984;131:1-8. [PubMed]
- Molina JR, Quevedo JF, Furth AF, et al. Predictors of survival from urachal cancer: a Mayo Clinic study of 49 cases. Cancer 2007;110:2434-40. [Crossref] [PubMed]
- Zhang J, Wu J. Options for diagnosis and treatment of urachal carcinoma. Asia Pac J Clin Oncol 2013;9:117-22. [Crossref] [PubMed]
- Ashley RA, Inman BA, Routh JC, et al. Urachal anomalies: a longitudinal study of urachal remnants in children and adults. J Urol 2007;178:1615-8. [Crossref] [PubMed]
- Herr HW, Bochner BH, Sharp D, et al. Urachal carcinoma: contemporary surgical outcomes. J Urol 2007;178:74-8; discussion 78. [Crossref] [PubMed]
- Herr HW. Urachal carcinoma: the case for extended partial cystectomy. J Urol 1994;151:365-6. [PubMed]
- Henly DR, Farrow GM, Zincke H. Urachal cancer: role of conservative surgery. Urology 1993;42:635-9. [Crossref] [PubMed]
- Grignon DJ, Ro JY, Ayala AG, et al. Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer 1991;67:2165-72. [Crossref] [PubMed]
- Yazawa S, Kikuchi E, Takeda T, et al. Surgical and chemotherapeutic options for urachal carcinoma: report of ten cases and literature review. Urol Int 2012;88:209-14. [Crossref] [PubMed]
- Siefker-Radtke A. Urachal carcinoma: surgical and chemotherapeutic options. Expert Rev Anticancer Ther 2006;6:1715-21. [Crossref] [PubMed]
- Siefker-Radtke AO, Gee J, Shen Y, et al. Multimodality management of urachal carcinoma: the M. D. Anderson Cancer Center experience. J Urol 2003;169:1295-8. [Crossref] [PubMed]
- Krane LS, Kader AK, Levine EA. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for patients with peritoneal carcinomatosis secondary to urachal adenocarcinoma. J Surg Oncol 2012;105:258-60. [Crossref] [PubMed]
- Kang M, Jeong CW, Kwak C, et al. Survival Outcomes and Predictive Factors for Female Urethral Cancer: Long-term Experience with Korean Patients. J Korean Med Sci 2015;30:1143-9. [Crossref] [PubMed]
- Paner GP, Barkan GA, Mehta V, et al. Urachal carcinomas of the nonglandular type: salient features and considerations in pathologic diagnosis. Am J Surg Pathol 2012;36:432-42. [Crossref] [PubMed]
- Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res 2012;18:1506-12. [Crossref] [PubMed]
- Sirintrapun SJ, Ward M, Woo J, et al. High-stage urachal adenocarcinoma can be associated with microsatellite instability and KRAS mutations. Hum Pathol 2014;45:327-30. [Crossref] [PubMed]
- Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer 2007;43:1529-44. [Crossref] [PubMed]
- Nakanishi K, Kawai T, Suzuki M, et al. Prognostic factors in urachal adenocarcinoma. A study in 41 specimens of DNA status, proliferating cell-nuclear antigen immunostaining, and argyrophilic nucleolar-organizer region counts. Hum Pathol 1996;27:240-7. [Crossref] [PubMed]
- Kipp BR, Tyner HL, Campion MB, et al. Chromosomal alterations detected by fluorescence in situ hybridization in urothelial carcinoma and rarer histologic variants of bladder cancer. Am J Clin Pathol 2008;130:552-9. [Crossref] [PubMed]
- Jordan E, Won H, Toubaji A, et al. Assessment of genomic alterations in bladder adenocarcinoma and urachal adenocarcinoma. Eur J Cancer 2015;51:S530. [Crossref]
- Torenbeek R, Lagendijk JH, Van Diest PJ, et al. Value of a panel of antibodies to identify the primary origin of adenocarcinomas presenting as bladder carcinoma. Histopathology 1998;32:20-7. [Crossref] [PubMed]
- Liu Q, Teh M, Ito K, et al. CDX2 expression is progressively decreased in human gastric intestinal metaplasia, dysplasia and cancer. Mod Pathol 2007;20:1286-97. [Crossref] [PubMed]
- Painter JT, Clayton NP, Herbert RA. Useful immunohistochemical markers of tumor differentiation. Toxicol Pathol 2010;38:131-141. [Crossref] [PubMed]
- Sanada Y, Oue N, Mitani Y, et al. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol 2006;208:633-42. [Crossref] [PubMed]
- Shariat SF, Zlotta AR, Ashfaq R, et al. Cooperative effect of cell-cycle regulators expression on bladder cancer development and biologic aggressiveness. Mod Pathol 2007;20:445-59. [Crossref] [PubMed]
- Kapur P, Lotan Y, King E, et al. Primary adenocarcinoma of the urinary bladder: value of cell cycle biomarkers. Am J Clin Pathol 2011;135:822-30. [Crossref] [PubMed]
- Niedworok C, Panitz M, Szarvas T, et al. Urachal Carcinoma of the Bladder: Impact of Clinical and Immunohistochemical Parameters on Patient Prognosis. J Urol 2015. pii: S0022-5347(15)05382-3.
- Szarvas T, vom Dorp F, Niedworok C, et al. High insulin-like growth factor mRNA-binding protein 3 (IMP3) protein expression is associated with poor survival in muscle-invasive bladder cancer. BJU Int 2012;110:E308-17. [Crossref] [PubMed]
- Zong L, Chen P. Surgical and chemotherapeutic experience regarding a urachal carcinoma with repeated relapse: case report and literature review. World J Surg Oncol 2013;11:170. [Crossref] [PubMed]
- Yoshida Y, Yamanaka K, Ueda N, et al. A case of urachal carcinoma with multiple lung metastases treated by TS-1/CDDP chemotherapy. Hinyokika Kiyo 2014;60:147-50. [PubMed]
- Lee W. Urachal adenocarcinoma metastatic to the ovaries resembling primary ovarian mucinous carcinoma: a case report with the immunohistochemical study. Int J Clin Exp Pathol 2010;4:118-23. [PubMed]
- Louhimo J, Alfthan H, Stenman UH, et al. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology 2004;66:126-31. [Crossref] [PubMed]
- Sundar S, Neal RD, Kehoe S. Diagnosis of ovarian cancer. BMJ 2015;351:h4443. [Crossref] [PubMed]
- Yanagihara Y, Tanji N, Miura N, et al. Modified FOLFOX6 chemotherapy in patients with metastatic urachal cancer. Chemotherapy 2013;59:402-6. [Crossref] [PubMed]
- Fluorouracil, Leucovorin, Gemcitabine, and Cisplatin in Treating Patients With Metastatic or Unresectable Adenocarcinoma of the Urothelium or Urachal Remnant [Internet]. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00082706?term=urachal+cancer&rank=1


