Lynch syndrome and exposure to aristolochic acid in upper-tract urothelial carcinoma: its clinical impact?
Introduction
Although urothelial carcinoma of the bladder is the fourth most common cancer worldwide, upper urinary-tract urothelial carcinomas (UTUCs) are rare, representing only 5% of genitourinary malignancies. In Europe, the estimated incidence of UTUCs is 1.2 cases per 100,000 individuals per year (1). Several etiological factors for urothelial neoplasia have been identified, of which the most frequent are tobacco and occupational exposure, which induce urothelial carcinoma in both the bladder and upper urinary tract (2).
Ingestion of aristolochic acid (AA) is now recognized to be a carcinogenic agent that causes severe renal disease and UTUCs in exposed populations worldwide (3-5). Similarly, LS [hereditary non-polyposis colorectal cancer (HNPCC)] increases the risk of developing UTUC by 14–22-fold compared to the general population (6,7).
In this brief review, we describe two specific environmental and constitutional factors involved in the genesis of UTUC and the clinical impacts of these etiologies.
Material and methods
A Medline search was performed with special emphasis on HNPCC and exposure to AA using combinations of the following terms: AA, Balkan nephropathy (BNe), hereditary cancer, HNPCC, mismatch repair genes, urothelial carcinomas, upper urinary tract, renal pelvis, ureter, Amsterdam criteria, genetic counselling, mismatch repair genes, genetic instability, microsatellite, and Bethesda guidelines. Articles were selected depending on the date of publication, the quality of the study, and their relevance to the theme of this review. Articles considered were published between 1995 and 2015 although older studies and particularly notable publications in this very specialist area were also included.
Results
Although the mechanisms of carcinogenesis were thought to be similar throughout the urinary tract, recent epidemiological data and genetic studies suggest otherwise. It is now obvious that strong differences exist for tumour location and behavior between the upper urinary tract and the bladder. To date many support the hypothesis that genetic susceptibility to cancer is enhanced by exposure to exogenous factors that induce specific-gene environment interactions and promote the development of UTUC (6,7).
Lynch syndrome (LS)
LS or HNPCC is the commonest monogenetic factor that predisposes to colorectal cancer and accounts for 1–3% of these tumors (7). This syndrome is divided into two types: type I corresponds to cases of colorectal adenocarcinoma in families that meet the revised Amsterdam criteria; and type II has the same as the criterion as type I plus its association with other extra-colonic cancers (endometrial, ovarian, upper urinary tract, small intestine, stomach, biliary tract, larynx, brain).
There are several variant subtypes of LS syndrome associated with different tumor localizations, described collectively within the “classic” spectrum (Muir Torre syndrome, with tumors of the sebaceous glands; keratoacanthomas of the papilla of Vater or biliary tract; and Turcot syndrome with glioblastomas) (8).
In LS, UTUCs are the third most common tumor localization (5–6%) after colon cancer (63%) and endometrium cancer (9%) (6). In LS, the relative risk of an individual developing UTUC varies from 14–22 (6,7).
There is a lack of appreciation of the association of LS and a wide variety of extra-colonic tumors: thus, some hereditary cancers are probably misclassified as sporadic and the incidence of LS may be underestimated.
Genetically, this familial colorectal cancer is characterized by an autosomal dominant transmission. LS is associated with a germline mutation of one of the six genes in the DNA-mismatch repair system (MMR): i.e., hMSH2, hMSH3, hMSH6, hMLH1, hPMS1, hPMS2 (6). This type of mutation is causes an unstable tumor phenotype that can be detected by assessing microsatellite instability (MSI). In the absence of a functional MMR system, tumor cells accumulate replication errors, particularly at non-coding nucleotide sequences (microsatellites). The repetition size varies from one cell to another (expansion or contraction). The MSI phenotype simply reflects the existence of this genetic epiphenomenon and is an indirect reflection of MMR malfunction (6).
Diagnosis of urinary-tract urothelial carcinoma (UTUC) in LS
The specific clinical characteristics of UTUC in cases of LS are the following (6,9): a female predominance (gender ratio: male/female =0.95), an average younger age at onset (55–60 years); a high rate of ureteral tumors (51%), and a family history of cancer within the LS spectrum. Conventionally, bladder tumors are not included in the “classic” LS tumor spectrum; however, recent work suggests an increased risk in this location, especially in cases when the MSH2 gene is mutated (10-12).
Clinical suspicion of LS is based on the existence of a colonic neoplasia (cancer or adenoma) in a familial context. According to the family history, the clinical criteria for guidelines were defined by an international consortium in 1991 and revised in 1999 (Amsterdam I and II criteria) (6,13). These criteria are highly selective and are used in epidemiological studies. However, some investigators suggest that the stringent Amsterdam II criteria may miss a significant number of patients with LS. Even if 60% of families presenting with these criteria have a LS mutation (good specificity), the negative predictive values are low, with 80% of families with LS not selected by these criteria (low sensibility) (7).
Other criteria, such as the so-called Bethesda criteria [2003], are less selective but are more sensitive and help clinicians detect more families at risk (14). According to these criteria, clinicians can define patients who are eligible for a genetic diagnosis. The European guidelines propose genetic testing in patients who are diagnosed when aged ≤60 years and in patients with UTUC with a personal or family history of LS (15). Thus, the French collaborative group on UTUC has proposed a screening tool for urologists to use during consultations (16).
Once a diagnosis of LS is suspected, according to the clinical data, clinicians could ideally request a search for MSI status for UTUC on specimens obtained via a nephroureterectomy or ureterectomy. However, these biopsy specimens are not feasible in practice as they require a comparison with healthy tissue.
The National Cancer Institute recommends the use of five microsatellite allele markers to assess MSI status (17). A high MSI tumor is defined by ≥30% shifted alleles, a low MSI tumor include has <30% shifted alleles, and a tumor with no shifted alleles is defined as MSI stable.
In cases where there is a high MSI status in UTUC, a hereditary predisposition to cancer should be assessed. Diagnostic confirmation by DNA sequencing is then carried out to search for a mutation in one of the MMR genes. An hMSH2 gene mutation is the most common (60%) in patients with LS and UTUC. Other mutations are also described, but at smaller proportions: i.e., hMLH1 (30%) and hMSH6 (5–8%). Mutations of other genes (hMSH3, hPMS1, and hPSM2) are less common (6). However, this sequencing technique is time consuming and expensive, although may be first guided by an immunohistochemical search of the UTUC specimen for a loss of expression of the proteins encoded by these genes (14).
Once a diagnosis of LS is established, the patient and his/her family should undergo an oncogenetic consultation to detect other possible LS-spectrum tumors. Even though UTUCs are rare, systematic screening of MSI in all UTUC specimens should be considered. In parallel, all colonic tumors analyzed detect only 1–3% of patients with LS.
As previously reported, UTUCs are found in 6% of LS patients. However, few published data are available concerning the frequency of LS in the UTUC population. In a recent study concerning 1,122 patients with UTUC, the authors reported that 21% of patients had positive Bethesda criteria (16). However, this percentage only reflects the total number of patients with genetically proven LS (i.e., only 20%) and who had positive criteria compared to non-restrictive and less sensitive criteria (14). Thus, today, to achieve optimal efficiency but also at a reasonable cost, it seems only fair to provide mutational analysis for all primary UTUC patients that meet the Bethesda criteria.
Apart from familial associations and the prevention of other tumors in this spectrum, a diagnosis of LS has a direct clinical impact on patients with UTUC. Indeed, in the context of LS (high MSI status), UTUC provides a better prognosis than tumors that occur sporadically in localized forms (≤T2N0M0) (18). In addition, as has been described for colon cancer, locally advanced or metastatic UTUCs in LS could be more sensitive to chemotherapy than sporadic cases (19).
Follow-up of LS patients
Few data have reported on the urological follow-up of LS patients (20). Published data report that those with familial LS and patients carrying a MSH2 mutation are particularly at risk of carcinogenesis of the upper urinary tract and so should be monitored closely (10-12). According to a recent review, assessment of the upper urinary tract could aid risk stratification (20).
Patients with LS need to be informed of the risk of UTUC and the necessity of complementary exploration in case of macroscopic hematuria. Regular urine analysis is recommended to detect microscopic hematuria. If the test is positive, this should lead to exploration of the upper urinary tract for gross hematuria (20).
LS patients usually undergo annual urine cytology, cystoscopy and urinary analysis; however, although these tests have the advantage of being inexpensive, their sensitivity is low to detect UTUC. At present, no consensus exists concerning imagery of the upper urinary tract (20). Conducting urography or obtaining a CT-scan is not legitimate when monitoring LS patients given the cost of examinations and the exposure to radiation; however, the regular use of renal and bladder ultrasound in the most at-risk patients can be an option.
Apart from urological monitoring, LS patients need to be followed-up with regular colonoscopies conducted earlier and more frequently than for individuals in the general population (21,22). The time of a first colonoscopy may vary depending on the mutated gene type and the median age at diagnosis of onset of colon cancer within the family. Moreover, chemoprevention (600 mg aspirin daily) has been proposed to reduce the risk of colon cancer in LS patients (23). In addition, a systematic hysterectomy has been proposed by national groups and groups of experts for women with LS (24).
Aristolochic acid (AA) and urinary-tract urothelial carcinoma (UTUCs)
Balkan nephropathy (BNe)
Since 1950, a remarkably high incidence of UTUC (60–100 times greater than the rest of the world) has been reported in some rural areas of the Balkans (Bosnia, Bulgaria, Croatia, Romania, Serbia) (25). However, there incidence been reduced over the past 20 years [only 11-times higher in endemic areas in 1998 compared to 57 times higher in 1988, according to Markovic et al. (26)].
These UTUCs have been associated with endemic nephropathy, termed BNe, which corresponds to proximal tubular dysfunction. It is caused by low molecular-weight proteinuria and interstitial fibrosis that affect the glomeruli. UTUCs in BNe have specific characteristics compared to sporadic UTUCs (27): i.e., these cases are more frequently bilateral (8–10%) than in the general population, there is no female to male predominance; the cases usually occur in rural areas; and, on average, UTUC is diagnosed about 10 years after BNe is diagnosed.
The etiologic agent for BNe has now been identified as Aristolochia clematitis (a Balkan endemic plant) (28). This plant grows in wheat fields, where it then contaminates flour, is made into bread and is then eaten by the local population. This plant, like other Aristolochia species (~500 species) contains AA, a nephrotoxic, mutagenic, and carcinogenic substance.
The metabolism of AA is very complex and remains partially unknown. Several liver and kidney enzymes are involved in detoxification of AA adducts. There is interindividual variability in the development of UTUC within Balkan endemic areas: this may be explained by gene polymorphism within the detoxification systems of each individual (including cytochrome p450 for toxic derivatives of AA) (29,30).
Chinese herb nephropathy
Another nephropathy associated with a high incidence of UTUCs has highlighted the role of AA in BNe. In Belgium, between 1992 and 1993, 43 patients were hospitalized for severe to end-stage renal failure following ingestion of Chinese herbal medicines that caused “Chinese herbal nephropathy” (CHN) (3-5). An etiological survey highlighted substitution of Stephania tetrandra (“han fang ji” in Pin Yin language) in medicinal mixture by Aristolochia fanchi (“Guang fang ji”). Similar to BNe, renal involvement in CHN corresponded to a specific tubulointerstitial nephritis that almost completely destroyed the renal cortex through interstitial fibrosis with a specific histological appearance. UTUC was diagnosed in 46% of poisoned patients.
The role of AA within Aristolochia was later confirmed in several studies (3-5). A specific mutation induced by active metabolites derived from AA was found at codon 139 in the p53 gene (AAG → TAG; Lys → Stop) (31). This mutation is predominant in patients with BNe or CHN, but is very rare in patients not exposed to AA but who have UTUC. Because of its specificity, this mutation is a fingerprint for exposure to AA.
A meta-analysis established an odds ratio of 5.97 (95% CI, 2.78–12.84) for developing UTUC after exposure to AA (5), and this risk may persist for many years (sometimes >10 years) after stopping exposure to AA (32,33). A high carcinogenic risk was observed after ingestion of at least 200 mg of AA (3,4). The tubulo-interstitial lesions induced by AA are also consistently associated with further development of urothelial cancer; this has been described in cases of UTUCs before degradation of renal function. Today, BNe and CHN are jointly termed “acid aristolochic nephropathy”.
Worldwide distribution of acid aristolochic nephropathy
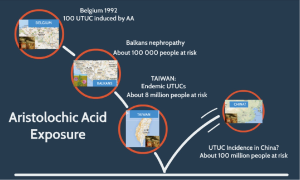
Acid aristolochic nephropathy is not only limited to the Balkan areas and Belgium (4,34) (Figure 1): some cases of acid aristolochic nephropathy have been reported in France, Spain, Germany, the UK, and the United States (4,35). Moreover, a growing number of cases have been described in Asia (Japan, Korea, China, Taiwan, Hong Kong); it is likely that acid aristolochic nephropathy has affected the whole Asian continent but has been underestimated in cases of chronic kidney disease and UTUC (4,36).
Seven species of Aristolochia (including Aristolochia fanchi) are used in Chinese medicine, and AA content varies with species. In Asia, the scientific names of medicinal herbs are rarely used in traditional medicines in favor of their vernacular names, which have allowed their substitution in medicines (34). This has caused health problems across the whole continent: for example, it is known that ~230 million Chinese annually use traditional medicines (34).
It has been estimated that one-third of Taiwanese people have ingested AA (37). This may be correlated with the dramatically high rate of UTUC (10–25% of all urothelial carcinomas) in Taiwan (38,39). This high incidence was previously thought to be caused by exposure to arsenic in polluted water from artesian wells (in specific areas this is known as “black foot disease”), but this association has never been formally proven (38,39). It is now realized that co-exposure to AA in Taiwan, along with other risk factors for UTUC (such as tobacco or arsenic) may also be a causative factor (40).
Other traditional medicines in India, Iran, Moroccan, and elsewhere, also use plants from the Aristolochia genus (e.g., A. bracteata, A. indica, A. tagala, A. baetica and A. longa) (41-43). It is therefore very likely that the incidence of tubulo-interstitial nephritis and the development of UTUC induced by AA exposure are underestimated public-health issues at a global level (4).
The use of species containing AA are prohibited by the Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Agency for Research on Cancer (IARC), which rank AA as class I carcinogen. There is now a consensus diagnosis on NAA made by a panel expert, which has combined the major and minor criteria (44). A diagnosis is certain when renal failure (glomerular filtration is <60 mL/min/1.73 m2) is associated with at least two other major criteria: i.e., hypocellular renal interstitial fibrosis with decreasing intensity according to cortico-medullary gradient; ingestion of products containing AA (defined by phytochemical analysis); and detection of specific DNA adducts in renal or urothelial tissular specimens.
A diagnosis is probable when only one major criterion is associated with two minor criteria: i.e., previous consumption (by alimentation or traditional medicine) of presumed products containing AA, and the presence of UTUC (or other urothelial carcinoma).
Physicians must be aware and suspicious when diagnosing UTUC in a context of atypical chronic kidney disease or clinical argument for LS. The use of so-called “natural medicine” or family history of LS spectrum tumors should be appropriately search during patient interviews. Dedicated criteria have been established for imputing each of these risk factors in UTUC carcinogenesis. In practice, the clinician suspects a case of UTUC in the context of atypical renal failure. In this setting, patients should be asked if they have previously ingested AA, and a renal biopsy remains a strong element of diagnosis because of the specificity of renal lesions. The positive clinical screening could lead to the achievement of specific analyses using immunochemistry or genetic assays when indicated, considering that the detection of these risk factors directly impacts the follow-up of these UTUC patients. The follow-up should be extended after treatment of UTUC because of the risk of bilateral lesions and their recurrence in the bladder long after exposure to AA.
Acknowledgements
None.
Footnote
Conflicts of Interest: SF Shariat owns or co-owns the following patents: Methods to determine prognosis after therapy for prostate cancer. Granted 2002-09-06. Methods to determine prognosis after therapy for bladder cancer. Granted 2003-06-19. Prognostic methods for patients with prostatic disease. Granted 2004-08-05. Soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma. Granted 2010-07-20. He is advisory board member of Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanofi, Wolff. He is speaker for Astellas, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, Wolff. R Mathieu: Consultant: Astellas, Ipsen, Janssen; Speaker: Janssen, Sanofi, Novartis, Takeda. The other authors have no conflicts of interest to declare.
References
- Visser O, Adolfsson J, Rossi S, et al. Incidence and survival of rare urogenital cancers in Europe. Eur J Cancer 2012;48:456-64. [Crossref] [PubMed]
- Rouprêt M, Colin P. Urothelial carcinomas of the upper urinary tract are now recognised as a true and distinct entity from bladder cancer and belong fully to the broad spectrum of onco-urologic neoplasms. World J Urol 2013;31:1-3. [Crossref] [PubMed]
- Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 2000;342:1686-92. [Crossref] [PubMed]
- Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int 2008;74:158-69. [Crossref] [PubMed]
- Wu F, Wang T. Risk assessment of upper tract urothelial carcinoma related to aristolochic acid. Cancer Epidemiol Biomarkers Prev 2013;22:812-20. [Crossref] [PubMed]
- Rouprêt M, Yates DR, Comperat E, et al. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (lynch syndrome) tumor spectrum. Eur Urol 2008;54:1226-36. [Crossref] [PubMed]
- Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851-60. [Crossref] [PubMed]
- Barrow P, Khan M, Lalloo F, et al. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg 2013;100:1719-31. [Crossref] [PubMed]
- Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer 2008;123:444-9. [Crossref] [PubMed]
- Joost P, Therkildsen C, Dominguez-Valentin M, et al. Urinary Tract Cancer in Lynch Syndrome; Increased Risk in Carriers of MSH2 Mutations. Urology 2015;86:1212-7. [Crossref] [PubMed]
- Crockett DG, Wagner DG, Holmäng S, et al. Upper urinary tract carcinoma in Lynch syndrome cases. J Urol 2011;185:1627-30. [Crossref] [PubMed]
- Skeldon SC, Semotiuk K, Aronson M, et al. Patients with Lynch syndrome mismatch repair gene mutations are at higher risk for not only upper tract urothelial cancer but also bladder cancer. Eur Urol 2013;63:379-85. [Crossref] [PubMed]
- Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. [Crossref] [PubMed]
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Compérat E, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 2013;63:1059-71. [Crossref] [PubMed]
- Audenet F, Colin P, Yates DR, et al. A proportion of hereditary upper urinary tract urothelial carcinomas are misclassified as sporadic according to a multi-institutional database analysis: proposal of patient-specific risk identification tool. BJU Int 2012;110:E583-9. [Crossref] [PubMed]
- Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248-57. [PubMed]
- Rouprêt M, Fromont G, Azzouzi AR, et al. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology 2005;65:1233-7. [Crossref] [PubMed]
- Hollande C, Colin P, de La Motte Rouge T, et al. Hereditary-like urothelial carcinomas of the upper urinary tract benefit more from adjuvant cisplatin-based chemotherapy after radical nephroureterectomy than do sporadic tumours. BJU Int 2014;113:574-80. [Crossref] [PubMed]
- Mork M, Hubosky SG, Rouprêt M, et al. Lynch syndrome: a primer for urologists and panel recommendations. J Urol 2015;194:21-9. [Crossref] [PubMed]
- Vasen HF, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013;62:812-23. [Crossref] [PubMed]
- Burt RW, Barthel JS, Dunn KB, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw 2010;8:8-61. [PubMed]
- Burn J, Mathers J, Bishop DT. Lynch syndrome: history, causes, diagnosis, treatment and prevention (CAPP2 trial). Dig Dis 2012;30 Suppl 2:39-47. [Crossref] [PubMed]
- Mills AM, Liou S, Ford JM, et al. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol 2014;38:1501-9. [Crossref] [PubMed]
- Bamias G, Boletis J. Balkan nephropathy: evolution of our knowledge. Am J Kidney Dis 2008;52:606-16. [Crossref] [PubMed]
- Markovic N, Ignjatovic I, Cukuranovic R, et al. Decreasing incidence of urothelial cancer in a Balkan endemic nephropathy region in Serbia. A surgery based study from 1969 to 1998. Pathol Biol (Paris) 2005;53:26-9. [Crossref] [PubMed]
- Colin P, Koenig P, Ouzzane A, et al. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int 2009;104:1436-40. [Crossref] [PubMed]
- Grollman AP, Shibutani S, Moriya M, et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci U S A 2007;104:12129-34. [Crossref] [PubMed]
- Stiborová M, Frei E, Schmeiser HH. Biotransformation enzymes in development of renal injury and urothelial cancer caused by aristolochic acid. Kidney Int 2008;73:1209-11. [Crossref] [PubMed]
- Chen B, Bai Y, Sun M, et al. Glutathione S-transferases T1 null genotype is associated with susceptibility to aristolochic acid nephropathy. Int Urol Nephrol 2012;44:301-7. [Crossref] [PubMed]
- Slade N, Moll UM, Brdar B, et al. p53 mutations as fingerprints for aristolochic acid: an environmental carcinogen in endemic (Balkan) nephropathy. Mutat Res 2009;663:1-6. [Crossref] [PubMed]
- Lemy A, Wissing KM, Rorive S, et al. Late onset of bladder urothelial carcinoma after kidney transplantation for end-stage aristolochic acid nephropathy: a case series with 15-year follow-up. Am J Kidney Dis 2008;51:471-7. [Crossref] [PubMed]
- Nortier JL, Vanherweghem JL. Renal interstitial fibrosis and urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). Toxicology 2002;181-182:577-80. [Crossref] [PubMed]
- Nortier J, Pozdzik A, Roumeguere T, et al. Aristolochic acid nephropathy ("Chinese herb nephropathy"). Nephrol Ther 2015;11:574-88. [Crossref] [PubMed]
- Lord GM, Cook T, Arlt VM, et al. Urothelial malignant disease and Chinese herbal nephropathy. Lancet 2001;358:1515-6. [Crossref] [PubMed]
- Fang D, Zhang L, Li X, et al. Risk factors and treatment outcomes of new contralateral upper urinary urothelial carcinoma after nephroureterectomy: the experiences of a large Chinese center. J Cancer Res Clin Oncol 2014;140:477-85. [Crossref] [PubMed]
- Hsieh SC, Lin IH, Tseng WL, et al. Prescription profile of potentially aristolochic acid containing Chinese herbal products: an analysis of National Health Insurance data in Taiwan between 1997 and 2003. Chin Med 2008;3:13. [Crossref] [PubMed]
- Tan LB, Chen KT, Guo HR. Clinical and epidemiological features of patients with genitourinary tract tumour in a blackfoot disease endemic area of Taiwan. BJU Int 2008;102:48-54. [Crossref] [PubMed]
- Yang MH, Chen KK, Yen CC, et al. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology 2002;59:681-7. [Crossref] [PubMed]
- Chen CH, Dickman KG, Huang CY, et al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomes. Int J Cancer 2013;133:14-20. [Crossref] [PubMed]
- Bhattacharjee P, Bhattacharyya D. Characterization of the aqueous extract of the root of Aristolochia indica: evaluation of its traditional use as an antidote for snake bites. J Ethnopharmacol 2013;145:220-6. [Crossref] [PubMed]
- Yamani A, Bunel V, Antoine MH, et al. Substitution between Aristolochia and Bryonia genus in North-Eastern Morocco: toxicological implications. J Ethnopharmacol 2015;166:250-60. [Crossref] [PubMed]
- Ardalan MR, Khodaie L, Nasri H, et al. Herbs and hazards: risk of aristolochic acid nephropathy in Iran. Iran J Kidney Dis 2015;9:14-7. [PubMed]
- Gökmen MR, Cosyns JP, Arlt VM, et al. The epidemiology, diagnosis, and management of aristolochic acid nephropathy: a narrative review. Ann Intern Med 2013;158:469-77. [Crossref] [PubMed]